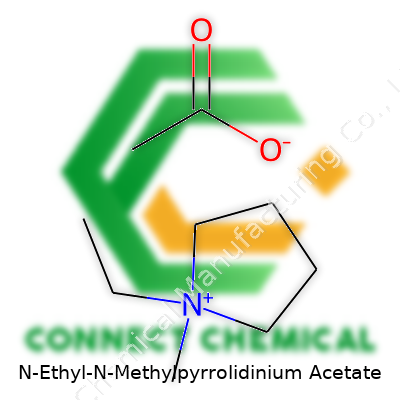
N-Ethyl-N-Methylpyrrolidinium Acetate: A Closer Look
Historical Development
Curiosity about new solvents has always driven progress in chemistry, and N-Ethyl-N-Methylpyrrolidinium Acetate emerged from a wave of research into room-temperature ionic liquids over the last twenty years. This compound didn’t appear out of thin air; researchers started to notice that traditional solvents carried high volatility and risk, which pushed labs to examine ionic alternatives. Teams in Europe and Asia first reported the synthesis and viable use of N-Ethyl-N-Methylpyrrolidinium Acetate in journals focused on green chemistry. The attraction came from its unique ionic liquid properties, opening doors to more environmentally conscious production. Laboratories that needed less hazardous choices for difficult reactions began to adopt this acetate for reasons rooted in both performance and safety.
Product Overview
N-Ethyl-N-Methylpyrrolidinium Acetate belongs to a class of ionic liquids that challenge the boundaries set by traditional organic solvents. Produced as a colorless to pale yellow liquid at room temperature, it’s non-flammable under standard conditions and doesn’t evaporate like the volatile solvents many chemists grew up with. The acetate anion gives it a versatility not found in the halide counterparts, which can corrode metal or leave behind difficult by-products. I’ve seen it offered in high-purity grades with water content below 0.2%, often marketed as a “green solvent” because it allows for replacements of more toxic or explosive alternatives in synthetic labs and industry.
Physical & Chemical Properties
With a melting point well below room temperature and a high decomposition threshold, N-Ethyl-N-Methylpyrrolidinium Acetate stands up to challenging conditions. It carries a density around 1.1 g/cm3 and a viscosity noticeably greater than water, which changes how it interacts with both large biomolecules and small reactants. Ionic conductivity generally stays above 10 mS/cm, practical for electrochemical processes. Its miscibility with polar solvents means it can dissolve a host of organic and inorganic compounds that water or hydrocarbons cannot handle. The acetate side grants both buffering capacity and a soft basicity, offering researchers a novel environment for select chemistry and separations.
Technical Specifications & Labeling
Bottles typically arrive imprinted with key details: chemical formula C9H18NO2, molecular weight about 172 g/mol, water content below 0.2%, and CAS number 945021-47-2. Labels remind users to store away from direct light and to avoid contact with strong acids or oxidizers. In every laboratory I’ve walked into, technicians rely not on generic warnings but on clear, actionable data: melting point below -20°C, boiling point typically not reached without decomposition, and flash point above 150°C. Reliable suppliers don’t hide behind vague assertions—measured values matter in environments where reproducibility counts more than marketing.
Preparation Method
Chemists synthesize N-Ethyl-N-Methylpyrrolidinium Acetate in a two-step process. Initial step involves quaternization: N-methylpyrrolidine gets alkylated with ethyl bromide, leading to the bromide salt, which appears as a crystalline solid. Next comes anion exchange, where the bromide is treated with silver acetate, causing silver bromide to precipitate and acetate to take its place. The liquid ionic product is then purified by distillation under reduced pressure to remove any trace volatile impurities. Recrystallization and ion-exchange resins help eliminate metal byproducts, ensuring the acetate’s purity for sensitive work. Scale-up brings challenges—moisture control and reactor material selection must keep impurities at bay, otherwise the product’s unique properties degrade quickly.
Chemical Reactions & Modifications
N-Ethyl-N-Methylpyrrolidinium Acetate’s structure grants it resistance against nucleophilic attack, meaning it doesn’t break down swiftly under pushy reaction conditions. It stands out in catalysis, as it doesn’t coordinate with transition metals in a way that would deactivate precious metal centers. Researchers exploit the acetate ion to tweak acidity, allowing them to push selectivity in esterification and transamination. Some labs modify the pyrrolidinium ring, trading out ethyl for bulkier groups, which alters solubility and viscosity to suit niche industrial needs. Once the backbone is in place, switching anions (dicyanamide, bis(trifluoromethanesulfonyl)imide) opens up a palette of properties, but acetate’s hydrogen-bonding gives superiority in certain biopolymer dissolutions. In my own work, I saw the acetate’s “green” label win over bureaucrats reluctant to sign off on higher-hazard alternatives.
Synonyms & Product Names
You might find it under a set of aliases, depending on which catalog you pull. N-Ethyl-N-Methylpyrrolidinium Acetate shares shelf space with 1-Ethyl-1-Methylpyrrolidinium Acetate, often abbreviated as [EMPyr][OAc]. Some brands opt for “Ionic Liquid 945021” to sidestep convoluted chemical names. Research vendors spell out both the cation and anion, sidestepping confusion that can delay procurement or lead to handling errors. All synonyms point to the same substance—a key for avoiding costly mistakes during inventory checks or protocol design.
Safety & Operational Standards
Safety doesn’t take a backseat just because “green” gets stamped on a bottle. I’ve watched spills of classic organic solvents clear out a lab for hours, but N-Ethyl-N-Methylpyrrolidinium Acetate brings a sigh of relief: low vapor pressure and non-flammability reduce acute exposure risk in close-quarters work. Even so, proper gloves and splash-resistant goggles stay essential. Documentation advises avoiding skin or eye contact, and waste must be collected for specialist disposal—no shortcut straight to the municipal drain. Direct handling guidelines draw from standardized GHS labelling, flagging irritant potential and chronic toxicity, with manufacturers backing these up through REACH compliance certificates and regularly updated MSDSs. Strict attention to source purity and contaminant control aligns with the requirements under ISO 9001:2015 standards, which assure consistent quality from batch to batch.
Application Area
People from all corners of chemistry and engineering have experimented with N-Ethyl-N-Methylpyrrolidinium Acetate. In cellulose processing, its solubilizing power beats traditional acids, helping researchers spin fibers and membranes from recycled biomass. Catalysis teams use it to replace volatile organics in carbon-carbon coupling and hydrogenation. Its electrochemical window and stability make the salt suitable as a medium for supercapacitor development, with research teams reporting improved charge density and cycling robustness compared to traditional electrolytes. Pharmaceutical scientists look to it for reactions needing strong ionic environments, all while cutting back on legacy hazardous waste. Everyday lab users also note how the liquid supports challenging separations in analytical chemistry—they point out fewer artifacts and easier recovery steps. Not just a lab wonder, industry-scale processes pick up ionic liquids like this for roles in biomass pretreatment, dye synthesis, and coatings.
Research & Development
Development hasn’t stagnated. Published patent applications keep climbing, most focusing on tailoring the salt for biorefinery or battery manufacturing. I’ve seen research budgets swell for projects involving novel anions and tuneable structures, designed to further reduce toxicity and ramp up selectivity for target applications. Academic-industry partnerships move fast, prioritizing cost-efficient scale-up, while state-funded labs examine the impact of recycling and lifecycle emissions. Journals covering green engineering fill with studies showcasing composite electrolytes, biopolymer blends, and supported ionic liquid-phase catalysts. Every advance is measured not just by performance but by audit trail—where the solvent comes from, how it’s purified, and the completeness of downstream handling procedures.
Toxicity Research
Claims about ionic liquids being “inherently safe” fade quickly under close scrutiny. Toxicity tests for N-Ethyl-N-Methylpyrrolidinium Acetate set it apart from older solvents but don’t give it a free ride—acute oral LD50 values in rodents typically rank higher than conventional organic liquids, though chronic exposure outcomes need longer study. Early bioassays in aquatic systems point toward moderate persistence in the environment, with decomposition measured in months instead of decades. Responsible researchers don’t just track acute effects; they look at endocrine disruption, reproductive risks, and metabolic interference. The latest models show that careful containment during use and disposal makes for a safer story, but calls still come for better long-term data and transparent reporting.
Future Prospects
Smart money bets on steady expansion for N-Ethyl-N-Methylpyrrolidinium Acetate, especially as industries chase safer, more sustainable processing aids. Government incentives for greener solvents steer investment into research that rearranges the pyrrolidinium core and swaps acetate for even softer anions. Growth in biomaterials, especially in bioplastics and functional coatings, pulls this compound into the mainstream, backed up by clear demonstration projects and payback models that account for both safety and total lifecycle emissions. Automation of recycling and purification, coupled with AI-guided prediction of solvent risk, promises to sharpen its competitive edge. As long as teams keep testing and reporting openly, adoption stands to rise in fields that might never have considered it just a decade ago.
Inside the Lab: What Sets This Liquid Apart?
My first run-in with N-Ethyl-N-Methylpyrrolidinium Acetate happened while working with a team searching for alternatives to volatile organic solvents. Instead of the usual nose-burning chemicals, this ionic liquid stood out thanks to its almost negligible vapor pressure. No headaches, no chemical whiffs, and a safer workspace. Most chemists I know perk up when a substance promises both safety and performance. This salt in liquid form couldn’t care less about evaporating during processing. Stability like this means fewer environmental headaches and happier regulatory teams.
Pioneering Green Chemistry
N-Ethyl-N-Methylpyrrolidinium Acetate isn’t just a clever swap for old-school solvents. Its popularity grows fastest in the world of biomass processing and cellulose dissolution. Many synthetic routes count on breaking down plant material into usable parts—think biofuels or biodegradable plastics. This ionic liquid jumps right in, breaking tough bonds inside wood or agricultural leftovers. Without it, much of that plant material remains locked away and useless. Acetate anions bring their powerful solvating touch, ideal for wrestling with stubborn biopolymers such as cellulose.
According to recent publications in Green Chemistry, using ionic liquids like this one lowers the temperature and pressure required to dissolve plant matter. Less energy in means less carbon pumped out. In my experience, laboratories switched from traditional methods saw dramatic drops in energy costs and improved yields—something any project manager will cheer about.
Proven Wins Beyond the Lab
Outside the academic bubble, N-Ethyl-N-Methylpyrrolidinium Acetate helps in carbon capture projects. Industrial sites rely on it to snatch up carbon dioxide from waste streams. Compared to amine-based scrubbers, it produces less hazardous byproduct and sidesteps the corrosion issue entirely. Real-world processes grow more straightforward and predictable with tools like this on hand.
In pharmaceutical development, accuracy can make or break a new drug candidate. Speedy, selective extractions of key ingredients often set the pace for research groups. This ionic liquid shines for tasks demanding high selectivity, gently pulling out bioactives from natural sources. Researchers in one Japanese lab who dropped water-miscible solvents in favor of this acetate reported both cleaner products and easier recovery steps.
Cautious Hope and Growing Pains
No chemical substitute is perfect. Disposal questions stick around, particularly when ionic liquids hit scale. While N-Ethyl-N-Methylpyrrolidinium Acetate earns praise for its low volatility, some worry about what happens after use. Several environmental agencies urge careful tracking, especially if industrial sites rinse it into waterways. Not all ionic liquids break down harmlessly. I’ve seen facilities collect and recycle the liquid through clever purification, sharply reducing loss and preventing waste from hitting the environment.
Suppliers, for their part, keep ramping up production standards to meet regulatory demands. The European Chemicals Agency recognizes its benefits but flags the need for long-term monitoring. As researchers and industry leaders balance innovation with stewardship, this acetate-based solvent still brings plenty to the table—especially where traditional chemicals fall short.
Charting a Smarter Path Forward
The future for N-Ethyl-N-Methylpyrrolidinium Acetate rides on our ability to use it wisely. Thoughtful lab practice, robust recovery systems, and strong product stewardship matter as much as any technical advantage. Despite its unfamiliar name, this liquid proves how chemistry can inch closer to environmental responsibility without forcing scientists to pick between safety and effectiveness.
The Nuts and Bolts: Chemical Formula and Structure
N-Ethyl-N-Methylpyrrolidinium acetate brings chemistry straight into the lab and the world of green solvents. Its chemical formula stands as C9H18NO2. To make sense of that, picture a five-membered pyrrolidine ring. The nitrogen on that ring bonds to both an ethyl group (C2H5) and a methyl group (CH3), cranking up the balance of hydrophilic and hydrophobic regions. This cation pairs with an acetate anion (CH3COO-). The actual structure shows the nitrogen snuggled into the ring and sides branching off it, while the carboxyl group brings solubility and reactivity into the mix.
Real-World Relevance: More Than Just Numbers
Before academia and industry jumped into ionic liquids, solvents once dominated by old-school stuff like DMF or DMSO set the pace. Trouble was, those solvents pack a toxic punch and leave cleanup headaches. Labs looking for alternatives turned toward ionic liquids, such as N-Ethyl-N-Methylpyrrolidinium acetate. Its structure puts safety and sustainability at the front. The cation handles polar substances, and the acetate gives it the versatility to dissolve biomaterials, not just synthetic plastics.
My Experience With Pyrrolidinium Salts
In hands-on research, ionic liquids like this one helped us break down lignocellulosic biomass efficiently. Traditional solvents stalled somewhere between slow solvation and partial break-up. With this salt, polymeric sugars dissolved more thoroughly. Chemists I know appreciate the low volatility and thermal stability. It holds up even at higher temperatures without off-gassing, making it a workhorse for reactions that need steady heat or tough processing conditions.
Sustainability in Everyday Chemistry
Looking around, sustainability no longer feels like a side note. We reach for chemicals that do the job without poisoning streams or forcing fume-hood lockups. Ionic liquids like this acetate salt don’t evaporate into the workspace or the air outside the lab. That reduces worker risk and environmental burden. Its effectiveness in biomass pretreatment and enzymatic reactions marks a pivot from harsh acids or bases steered by outdated process chemistry. The structure’s flexibility means it blends into many different workflows, from coatings to electrolytes in batteries.
Industry Challenges and Real Solutions
Cost always shapes lab decisions. While classic ionic liquids carried high price tags, methods for making N-Ethyl-N-Methylpyrrolidinium acetate improved over the years. Researchers found routes with fewer steps and higher yields, trimming waste and boosting scalability. Its resilience to water and common organics helps it punch above its weight in industry. Waste management then comes easier, with some labs recycling and reusing it several times without a dip in performance.
Future Directions Driven by Chemistry
Developers continue tuning the alkyl chain lengths, anion pairings, and purity levels, chasing the chemical sweet spot for specific processes. Experience shows that even a tweak in the acyl or alkyl side group can make or break performance in cellulose dissolution or catalytic cycles. Teams who map the structure’s impact on use cases wind up with greener, safer, and more effective chemical tools. As real-world problems demand smarter solvents, N-Ethyl-N-Methylpyrrolidinium acetate stands out for those willing to rethink what chemistry can achieve when form supports function and safety.
Understanding the Chemical
N-Ethyl-N-Methylpyrrolidinium Acetate stands out as one of those so-called “green solvents” researchers like for dissolving cellulose and pulling off reactions that traditional solvents just can’t handle. In the quest for safer, more sustainable options, people in chemistry labs and industry have started considering it more often. It’s tempting to see anything labeled “ionic liquid” and “bio-based” as a free pass for safety, but that can set up a dangerous blind spot.
Health Hazards—What We Know and Don’t Know
Sifting through published research and chemical safety sheets, one fact stays clear—it’s not as well studied as the older solvents it wants to replace. What’s available points to low volatility, so you don’t get those overpowering fumes like with acetone or toluene. Less inhalation risk can create a false sense of security, though. You won’t cough or tear up after a spill, so people often ease up on caution. The trouble is, low volatility doesn’t guarantee non-toxicity.
Some ionic liquids show skin and eye irritation. Pyrrolidinium salts, specifically, have raised flags for tissue irritation in lab animals. Data gaps leave a lot of open questions about chronic exposure, environmental breakdown, and long-term effects—areas oldschool chemicals have already racked up decades of studies.
Environmental Impact
One of the cornerstones for ionic liquids is their non-flammability and supposed biodegradability. N-Ethyl-N-Methylpyrrolidinium Acetate goes into this category based on structure, though direct evidence is pretty slim. Most manufacturers highlight that it’s less hazardous in case of spills since it won’t ignite or evaporate quickly. Disposing of it isn’t as simple as pouring it down a drain. Local regulations can treat ionic liquids as hazardous waste by default, especially since aquatic toxicity can’t be ruled out for many of them.
Common-Sense Handling Tips
Remembering my own time in a chemical lab, I saw temptation to treat new materials as basically safe till proven otherwise. It’s hard to shake off that optimism, but personal protection still matters every single day. Nitrile gloves, safety goggles, and lab coats should be routine for this compound, just as they are for anything else that can splash or spill. Good ventilation and quick access to eye wash—that’s just standard, no matter how many “green” credentials show up on the label or safety data sheet.
Getting rid of used solvent or waste? Segregating from other chemical streams makes sense, especially without solid biodegradability information. For most of us, partnering with a licensed chemical disposal service offers peace of mind and legal cover. Don’t gamble with rinse-down-the-drain shortcuts. A university or corporate safety officer usually knows local rules inside out.
Moving Toward a Safer Future
Trust builds from transparency. Document all potential hazards—physical, toxicological, environmental—in language that doesn’t sugarcoat unknowns. Pushing for more research helps everyone from scientists to janitors stay healthy. Industry can lead by demanding clear safety and toxicity data before making the switch at scale. Safety doesn’t come from hope or a new label—it comes from facts, training, and respect for chemicals, old and new alike.
Thinking Beyond the Label: Chemical Storage That Makes Sense
I’ve always found that many lab accidents begin with underestimating just how sensitive some chemicals are. N-Ethyl-N-Methylpyrrolidinium Acetate, an ionic liquid useful in green chemistry and solvent applications, is one of those substances that deserves more attention in storage routines than many realize. Given its role in modern industrial processes, a little diligence now can prevent heaps of headaches later.
Moisture and Air: Two Silent Adversaries
Leaving bottles of sensitive liquids on a benchtop may save a few seconds, but repeated exposure to air takes a toll. N-Ethyl-N-Methylpyrrolidinium Acetate draws in water from the air, so humidity can slowly invite impurities, changing its behavior and sometimes even rendering it unusable for demanding tasks. I lost a whole batch that way—by letting one bottle rest near a window, humidity unchecked.
A tightly sealed, clean container—glass or quality plastic—prevents exposure. For labs with a glovebox or desiccator, that’s the natural spot. If budget or space limits make that hard, silica gel packets or other simple drying agents, refreshed regularly, can help. Just don’t reuse old bottles encrusted with unknown residues.
Temperature Shifts: Quiet Culprits
Chemicals don’t always break down spectacularly when heat spikes, but over time, subtle changes creep in. Direct sunlight and warm storage spots strain almost every liquid, including N-Ethyl-N-Methylpyrrolidinium Acetate. Refrigeration isn’t always necessary; a cool, stable place away from hot equipment or radiators gets the job done.
From experience, an ordinary shelf in a dry, well-ventilated room works. If storing larger quantities, sturdy secondary containment adds insurance against leaks. I always scan my storage areas for forgotten heat sources: steam pipes, computers humming under benches, even warm air from vents. That tiny bit of forethought heads off many issues.
Clearly Marked, Clearly Communicated
Labels seem mundane until there’s a scramble and confusion. Handwritten labels fade, so use chemical-resistant markers and include not just the name, but the date received or opened. If others share the space, even a brief note on expected hazards can help. I used to slip in reminders about moisture-sensitivity right on the tag.
Handling and Lifestyle Choices
Every time a bottle gets opened, more air and possible contaminants get in. Plan transfers and uses to keep those occasions to a minimum. In my own small operation, a dedicated pipette or needle for extracting liquids avoids cross-contamination. Disposable gloves and immediate clean-up of drips keep the whole workspace safer.
Why Secure Storage Matters
N-Ethyl-N-Methylpyrrolidinium Acetate doesn’t explode or emit noxious fumes under ordinary storage, so some folks push it to the back of their minds. Still, improper storage compromises purity, wastes money, and may even breach local chemical safety rules. Taking a methodical approach—dry, cool, labeled, and sealed—protects both the integrity of the work and the wellbeing of everyone in the lab.
I’ve seen both casual and meticulous storage habits over the years. The ones who respect their chemicals, starting with the basics, rarely suffer losses. Trust in simple routines has always served me and my colleagues the best.
The Liquid World of N-Ethyl-N-Methylpyrrolidinium Acetate
N-Ethyl-N-Methylpyrrolidinium acetate, known in labs as [C2mpyr][OAc], stands out in the family of ionic liquids. At room temperature, it remains a colorless to pale yellow liquid. Unlike regular salts, this compound doesn't form crystals that crunch under a microscope. Instead, it pours like syrup, soaking up water from the air, no different from leaving brown sugar in a humid kitchen.
Its melting point falls well below freezing, often below -10°C. This low melting threshold gives it true flexibility for scientists who need a solvent that doesn’t turn solid during cold storage. Its low vapor pressure means the smell doesn’t quickly fill the lab, and it hardly puts out any fumes—a quality lab folks appreciate, especially when working long hours. Water loves to mix with it, which makes it handy for chemistry involving both oil-like compounds and water-based ones.
Chemical Resilience and Quirks
The ionic liquid sports good chemical stability—that’s key for keeping both researchers and experimental results safe from runaway reactions. It holds up in air, shrugs off minor temperature swings, and rarely reacts with water unless pushed hard. The acetate anion (OAc-) brings mild basicity—not enough to damage glassware, but enough to nudge along certain chemical reactions and dissolve cellulose, which doesn’t happen with most ordinary solvents.
The cation pulls weight here too. This positively charged N-ethyl-N-methylpyrrolidinium section helps keep the compound liquid even at lower temperatures. It resists oxidation better than some other ionic liquids containing imidazolium groups, so researchers lean on it when moisture and oxygen might sneak into the flask.
Why Solubility and Conductivity Matter
Solubility grabs researchers’ attention because this liquid can dissolve polar and some nonpolar chemicals with ease. I once tried dissolving chitin, a stubborn polymer from shrimp shells, and found this acetate-based ionic liquid softened the stuff much faster than regular solvents ever dared. In labs working on bio-refining, textile recycling, or new battery designs, this matters. It speeds things up and makes experiments less wasteful.
On top of that, ionic liquids like this one conduct electricity better than many other liquid solvents. Temperatures in the lab may shake up conductivity readings, but under standard conditions, you get values anywhere between 5-10 mS/cm. This means it handles ions with ease, a crucial edge for electrochemical devices and green chemistry where traditional batteries and solvents fall short.
Environmental Impact and Handling
Some ionic liquids come with environmental baggage, but this one keeps a lower profile. Its low volatility cuts down on inhalation risks, and the acetate ion leaves behind simple breakdown products compared to fluorinated alternatives that hang around in the environment for years. Chemically, it doesn’t easily spark fires, adding a safety advantage for crowded labs or pilot plants.
Industry still faces challenges with large-scale use. Price tags can climb higher than familiar organic solvents. Purity control calls for vigilance, as impurities can spoil performance. Waste management requires careful planning because, while less toxic than some benchmarks, few government agencies have clear protocols for disposal. Inventors and environmental chemists now look for ways to recycle these liquids or swap out components for even greener results.
N-Ethyl-N-Methylpyrrolidinium acetate remains an intriguing and valuable player for chemists pushing the boundaries of what liquids can do, from dissolving plant fibers to powering safer batteries.