N-Ethyl-N-Methylpyrrolidinium Bromide: The Building Block with Broad Reach
Historical Development
N-Ethyl-N-Methylpyrrolidinium bromide didn't pop up overnight. Its roots go back to the curiosity of chemists exploring quaternary ammonium salts in the late 1900s. Back then, research into ionic liquids and niche solvents inspired synthesis routes that gave rise to this compound. Academic labs dived in, looking for materials that break the mold beyond classic solvents. The automotive and electronics boom brought extra energy to this field, with companies eager for robust, thermally stable substances. Over time, the story of this molecule changed from fringe curiosity to reliable tool across multiple industries.
Product Overview
Here we're dealing with a colorless to off-white, sometimes crystalline powder. The compound often comes in a highly purified form, its consistency making it workable in both lab and production environments. Chemists trust its stability and low volatility, appreciating how it mixes well with polar solvents. You find it sold under a range of trade names, often abbreviated to EMIBr, and every reputable supplier backs their claims with thorough purity data. Handling doesn't usually require exotic precautions, especially compared to nastier bromides out there.
Physical & Chemical Properties
This salt has a melting point floating around 180°C, demonstrating pretty strong thermal stability. The odor is faint or absent, which improves comfort in the lab. It's soluble in water, ethanol, and other common polar solvents, driving versatility in different processes. Its quaternary ammonium cation lends itself to strong electrostatic interactions, which matters in both organic synthesis and electrochemical setups. Density and refractive index land right where you'd expect for this class, and as a bromide salt, it keeps ionic conductivity reliable. Shelf life holds up well, provided moisture gets kept out.
Technical Specifications & Labeling
Commercial packaging typically lists purity above 98%, water content below 1%, and clear labeling for both CAS and batch numbers. Labels comply with GHS requirements: a clear hazard pictogram if needed, risk phrases, and instructions for storage. Reputable producers offer a certificate of analysis with every batch—critical for traceability. Label information enables quick identification, which matters on a crowded chemical shelf or in a regulatory audit. Shipping regulations tend to treat it as a low-risk solid, but paperwork still gets attention due to the bromide ion.
Preparation Method
The standard synthetic route starts with N-methylpyrrolidine, which undergoes ethylation using a bromoalkane, most often ethyl bromide. This quaternization happens in an aprotic solvent—acetonitrile or acetone as the go-to. Reaction proceeds with stirring at elevated temperatures, generally tracked by TLC. Excess solvents are stripped out post-reaction, and the residue crystallizes. Recrystallization from ethanol further sharpens purity. I remember carrying out similar reactions during grad school; cleanup sometimes dragged, but the underlying chemistry performed as advertised. For industrial quantities, continuous flow reactors cut down reaction times and improve yield.
Chemical Reactions & Modifications
Synthetic chemists appreciate the cation's stability under a range of conditions. Still, the bromide anion can exchange with other halides in metathesis, customizing final properties. The cation doesn't give up its alkyl groups easily, but it can participate in reactions with strong nucleophiles—for example, forming ionic liquids on further reaction with bulky anions. Adding bulky groups brings new functions, expanding the toolkit for battery electrolytes and solvent science. From my lab time, swapping the bromide for tetrafluoroborate unlocks some nifty conductivity gains.
Synonyms & Product Names
Science and business rarely stick to one name. N-Ethyl-N-Methylpyrrolidinium bromide picks up alternate monikers like EMIP-Br, 1-ethyl-1-methylpyrrolidinium bromide, and sometimes gets indexed under its IUPAC designation. Each catalog tends to settle on a favorite alias, which demands cross-checking CAS: 4611-67-0. In my own records, I've spotted creative abbreviations that made deciphering old research a scavenger hunt.
Safety & Operational Standards
Safety and health teams want a low-toxicity profile, and this compound typically obliges within the limits of responsible use. Proper lab gloves and goggles cut down exposure risk, especially during powder handling. Keep it away from moisture and sources of ignition. Inhalation should be avoided, not because of acute hazard, but plain good practice. Emergency protocols are straightforward, dominated by cleanup of spills using inert material. Storage in cool, dry places extends shelf life. Institutions maintain up-to-date Safety Data Sheets as the backbone of lab management. From personal experience, a clean and labeled container avoids mix-ups—these salts tend to look the same as their cousins.
Application Area
Industries value this molecule for its contribution to advanced materials, particularly as an ionic liquid precursor. Electrochemistry labs exploit its ionic character in batteries, fuel cells, and supercapacitors. Pharmaceutical researchers use it in phase-transfer catalysis, finding it speeds up stubborn reactions. In my former lab, we mixed EMIBr into gel electrolytes for next-gen sensor prototypes. Electronics manufacturers employ it for antistatic coatings; academic chemists harness it for specialty solvent exploration. The solar cell community, always hungry for new electrolytes, runs screening runs with pyrrolidinium bromides to edge out incremental efficiency. Water treatment companies look at these kinds of salts for selective ion removal—experiments continue, pilot plants scale up when things look good on paper.
Research & Development
Curiosity still drives much of the research around N-Ethyl-N-Methylpyrrolidinium bromide. Labs push the envelope by pairing it with new anions for custom ionic liquids, chasing higher conductivity and lower viscosity for green energy tech. Some studies dive into the solvent effect on organic transformations, while others focus on membrane development for separation science. Collaborative projects emerge between universities and industry partners, blending theory with gritty real-world testing. Funding bodies jump on board where sustainability and innovation align. Presentations at ACS conferences show how this molecule seeds next-gen devices and streamlined workflows.
Toxicity Research
Long-term health risks always draw scrutiny. So far, animal testing and in-vitro screens rate its acute toxicity as low, with no big red flags for mutagenicity or chronic exposure. Accumulation in the environment remains under review, because bromides can disrupt some ecosystems. Biodegradation studies show slow breakdown, raising responsible disposal issues. Regulatory agencies call for regular updates as new data rolls in. Handling protocols err on the side of caution. Regular glove use, proper waste containers, and air filtration stay in place until the whole picture comes in. Speaking from lab experience, I’ve watched researchers stay vigilant regardless of the initial safety score.
Future Prospects
N-Ethyl-N-Methylpyrrolidinium bromide still stands at the threshold of broader opportunity. Clean energy pushes mean demand for new ionic liquids won't slow—the battery and capacitor industries keep hunting for safer, better-performing electrolytes. Environmental questions will nudge makers to refine synthesis for greener, less wasteful protocols. Tighter regulations for bromides may show up, so forward-thinking labs look at integrating more recovery and recycling steps. Startups seize on this molecule for niche fermentation aids and advanced separation tech. With active research and public focus on sustainable chemistry, its role in new materials, clean processes, and medical technology seems set to grow.
Why Researchers Value This Compound
Scientists need tools that let them dig deeper into problems, and N-Ethyl-N-Methylpyrrolidinium Bromide provides one of those tools. In labs focused on chemistry and material science, this compound has picked up attention, especially for its ability to act as an ionic liquid or electrolyte. These ionic salts don’t behave like table salt—at room temperature, they can become liquids. That gives researchers a chance to experiment with reactions in a totally different way compared to water or oil-based systems.
I’ve watched people search for better conductors or solvents that don’t corrode equipment or catch fire at the first spark. This compound checks some big boxes. Its structure—containing both methyl and ethyl groups attached to pyrrolidinium—makes it stable, which helps it hold up under tough experimental settings. In electrochemistry setups, stability means less unwanted side reactions and a cleaner signal when chasing new battery technologies or advanced sensors.
Modern Applications and Real Risks
The push for safer, longer-lasting batteries has brought this compound into sharper focus. Unlike some earlier liquid electrolytes—which can break down and even explode—pyrrolidinium-based salts handle heat and charge cycles calmly. That’s one reason research around lithium-ion and sodium-ion batteries tests these newer salts. Better batteries aren’t just about making phones last longer; they make electric cars and backup power safer, too.
At the same time, there’s a real need for caution. Not every new chemical is friendly to people or the environment. Many salts, even ones that seem simple, can trigger skin irritation, breathing issues, or worse if handled carelessly. Responsible labs keep up with safety data, and plenty of peer-reviewed studies track toxicity and breakdown in real ecosystems. Balance lies in staying curious yet careful with scale-up.
Potential for Greener Technologies
Manufacturing is under increasing pressure to swap out hazardous solvents in old-school processes. N-Ethyl-N-Methylpyrrolidinium Bromide steps in as a possible replacement for volatile organic compounds in some reactions. It dissolves a surprising range of starting materials and supports chemical transformations that otherwise need stronger, riskier solutions.
I’ve heard from small startup founders who look for any edge to reduce overheads, cut insurance costs, and earn a clean-tech badge. If safer salts can keep things simple without sacrificing results, industry pays attention. Adoption isn’t automatic. Price, supply chain interruptions, and regulatory reverberations all play a part. It’s a chemical with promise, but solid planning and frequent quality checks matter just as much as scientific curiosity.
The Road Ahead
Research won’t slow down, and the next decade could see a jump from lab-scale innovation to factory floors. Engineers and policymakers keep nudging industry towards lower-emission, less hazardous manufacturing. Every time a group tests a new material like N-Ethyl-N-Methylpyrrolidinium Bromide and shares results in open-access journals, others learn and improve, tweaking recipes for more sustainable production. The hope is for safer batteries, cleaner solvents, and industries that can answer tough environmental questions with hard data and pragmatic trials.
Pulling Apart the Name
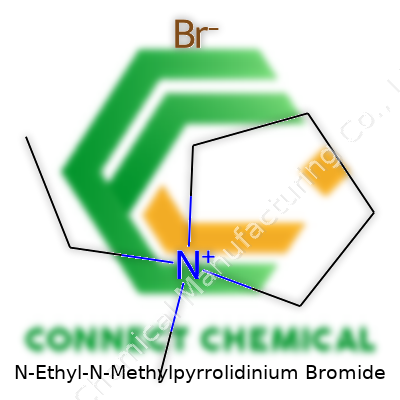
N-Ethyl-N-Methylpyrrolidinium Bromide carries a long name, but it breaks down into distinct pieces that reveal a lot about its identity. Start with pyrrolidine, a five-membered ring with four carbons and a nitrogen. This ring forms the backbone. Two substituents—an ethyl group and a methyl group—attach to the nitrogen, making the molecule a quaternary ammonium salt. The bromide part comes from bromine, joined as the counterion. In practice, you’ll see this structure described with a chemical formula: C7H16BrN.
Mapping the Structure
Imagine the pyrrolidine ring: a pentagon with one corner swapped out for a nitrogen atom. On this nitrogen sits both an ethyl group (–C2H5) and a methyl group (–CH3). Each provides bulk and influences the properties of the molecule. That nitrogen, now pushed into a positive charge by having too many bonds, grabs onto a bromide ion (Br–), which balances the charge.
Chemists often sketch this out for clarity. The nitrogen in the ring shares its electrons with the substituents, leaving a positive charge. Methyl and ethyl substituents point out from the ring, with the bromide ion hovering nearby, drawn in by the opposite charge. The presence of that positive charge means you’re not looking at a neutral molecule. Instead, you have a salt—something that acts very differently from neutral organic chemicals.
Where Chemistry Meets Everyday Life
People might overlook compounds like N-Ethyl-N-Methylpyrrolidinium Bromide, but the structure makes it useful in certain applications. For chemists, quaternary ammonium compounds often help dissolve tough-to-handle materials, drive reactions, or stabilize systems. The structure here allows for solubility in water but also flexibility in solvents that dissolve organics. In labs where people search for safer, less toxic materials compared to older solvents, these compounds show up again and again.
Looking out into the chemical industry, the usefulness centers on the way the ring and the positive charge influence interactions with other substances. Some scientists use this kind of molecule as an ionic liquid ingredient. By tuning the size and shape of those side chains—the ethyl and methyl groups—the properties shift in terms of melting point, solubility, and stability.
Balancing Promise and Caution
While the structure opens doors, it brings challenges. The bromide ion can add corrosiveness if not handled carefully. Ionic liquids, including salts like this one, sometimes push environmental boundaries—good solvents on one hand, but their stability means spills hang around. Researchers at universities and green chemistry labs run studies to find out where the molecules go after use, a key piece for sustainable chemistry.
Building Better Solutions
From first-hand lab experience, cleanup gets easier when you understand exactly what hangs off the nitrogen and how it moves within mixtures. Chemical literacy cuts down on risk. Instead of treating everything as the same, people can tailor handling and storage for these compounds. Regulators and manufacturers push for full disclosure of these structures in their supply chain, making sure people stay informed about what enters products.
Step back from the formula, and you see a story in the structure: a simple nitrogen ring, tuned by small changes, shapes the physical world in the lab and beyond. By keeping facts front and center, and listening to what science and safety research show, people can better manage the promise and potential risks of these distinctive molecules.
Why Storage Methods Matter
Over the years, I’ve seen how poor storage choices can turn a promising material into a headache. Plenty of good research has been sidelined because someone left a sensitive compound out in the wrong spot. One story sticks in my memory: A colleague set a container next to a heat register, forgetting the compound reacts badly to warm temperatures. By morning, the contents had turned brown and useless.
Heat, light, air, and moisture—these four troublemakers cause more waste and danger than almost anything else in a chemical storeroom. A lot of compounds break down or even turn hazardous when they meet water or oxygen, and sunlight speeds up those unwanted changes. The lesson? Pay attention to the small print on the label and don’t guess about where to stash something new.
Common-Sense Steps for Keeping Stock Safe
Dry containers with tight lids go a long way. Don’t assume a quick twist of the cap keeps air out; seals wear out. Desiccators work well for hygroscopic compounds (those that love to soak up moisture). Some powders seem harmless until you notice crystals forming on the rim—those crystals mean air or dampness has started a reaction.
Cool and dark spaces do wonders for compounds sensitive to heat and light. A lot of academic and industry labs rely on simple refrigerators for this reason. Dedicated, explosion-proof fridges stop flammable vapors from reaching a spark. At home or on a tight budget, the garage method doesn’t cut it. Even a locked box or insulated cooler in a quiet closet offers better control.
Labels should show not only what’s inside, but the opening date and who last handled it. Over time, dust and small spills add up, making labels unreadable. I once found an unlabeled canister that looked harmless, but turned out to contain an old peroxide-former. If someone had wiped it clean and labeled it, a dangerous situation never would have developed.
Why Handling Procedures Save More Than Product
Gloves, goggles, and lab coats seem basic, yet I’ve watched even experienced folks scoop powders with bare hands, trusting that “it won’t hurt me this once.” Repeated small exposures have real health effects. Imagine a powder so fine it floats through a sunbeam—each breath pulls in tiny particles that don’t just disappear.
Pouring or weighing out small amounts creates dust or spills. Local exhaust, like a fume hood, stops those clouds from spreading. After use, keeping benches and balance areas clean stops accidental contamination of similar-looking substances.
A chemical’s stability changes with age and use. Regular checks for color shifts, odd smells, or caking can warn you that it’s time to retire a batch. It pays off to keep records, not only for safety but also for audits and surprise inspections. Nothing ruins a routine day like a visit from the fire marshal followed by an argument about unlabeled or expired stock.
Practical Solutions Anyone Can Use
Education beats fancy gear every time. Everyone in the lab or workplace needs to know which compounds need special treatment—not just the supervisor. Posters help, checklists help even more. Grouping reactive or sensitive materials away from acids, bases, and flammables lowers the risk for accidents. If you’re short on space, use deep shelves with barriers, never stack containers on top of each other, and keep only what you need for current projects.
Disposal matters just as much as storage. Leftovers and expired materials can spark fires or create toxic gases if tossed out like regular trash. Find out what local regulations say, and keep the waste containers separate. I’ve seen bins fill up with old compounds, only for a new person to dump a reactive in on top. A good training session solves that before trouble starts.
Countless labs and businesses last longer and run safer because they respect these simple steps. It’s not fancy or expensive, but it works—every time.
What Is This Chemical, Really?
N-Ethyl-N-Methylpyrrolidinium Bromide often finds use in labs and some specialty industrial settings. You won’t see it on store shelves or find folks talking about it at dinner, but it comes up in the context of solvents, electrolytes, or research. To many, it looks like just another long chemical name until you think about the real-world implications of handling and exposure.
Is It Hazardous to Humans?
When researchers work with substances that contain a pyrrolidinium base, risks get more complicated than with the household cleaners most people know. Reports point out this compound acts as an irritant. It can get in through the skin or the eyes and cause discomfort. Inhaling dust or mist might trigger respiratory irritation or coughing fits. Swallowing some can upset your stomach at the very least.
I recall a graduate student in our chemistry department who didn’t take her gloves off before rubbing her eye. She experienced redness that lasted for hours. Even if something doesn’t burn a hole through a glove, good judgment says you keep your hands covered and don’t rush. Pyrrolidinium salts like this one should never be treated like sodium chloride.
Digging Into the Science of Toxicity
What sets this compound apart isn’t just lab inconvenience; there’s a reason chemical safety sheets highlight risks. N-Ethyl-N-Methylpyrrolidinium Bromide has a cationic nature and a bromide anion, so if it gets into the body in quantity, you don’t want to ignore it. While large-scale chronic exposure studies in humans remain sparse, data from relatives in the ionic liquid family show there’s potential for organ toxicity at higher concentrations, especially to the liver or kidneys. In studies with rodents, doses above recommended levels led to noticeable distress and, in some cases, organ changes. None of this spells everyday disaster for occasional splashes, but regular, unprotected exposure adds up over time.
Many chemicals like this one hang around in the environment longer than expected. While pyrrolidinium compounds usually don’t break down right away, persistent exposure in water or soil can accumulate, possibly affecting aquatic life or microorganisms at the bottom of the food chain. Local waterways deserve monitoring if this compound appears anywhere near drains or disposal systems.
Staying Safe and Smart in the Lab
Most labs require gloves, goggles, and fume hoods. These aren’t just for show. Ventilation and proper waste handling go a long way. From my own experience, labs that cut corners end up with more spills, sick days, or stress later. Teaching new students about real consequences—rather than treating everything like a generic “toxic hazard”—keeps trouble at bay.
Building a culture of respect for these lesser-known chemicals helps prevent overfamiliarity. Proper storage, regular checks, and clear labeling mean anyone grabbing a bottle later is less likely to make a big mistake. If waste sticks around, using sealed containers helps, and clear communication with hazardous materials disposal teams can save the water supply from unnecessary contamination.
How Do We Move Forward?
Anyone developing processes with N-Ethyl-N-Methylpyrrolidinium Bromide should keep up with the latest safety data and encourage transparency. Substituting safer alternatives where possible seems smart, though sometimes this compound brings something unique to the table. Better safety training and stronger reporting systems protect both people and the environment. Regulators and companies do well to expand long-term toxicity research and track waste outside the lab. Person-to-person responsibility remains at the heart of avoiding future hazards—each lab worker, each company, each disposal manager makes the difference.
Digging Into the Details
In labs and factories, N-Ethyl-N-Methylpyrrolidinium Bromide often stands out for its use as an ionic liquid component, phase transfer catalyst, or electrolyte. Scientists and industry professionals expect clear figures when it comes to handling chemicals. The molecular weight is a number that tells how much one mole of this compound weighs—usually about 222.13 g/mol for N-Ethyl-N-Methylpyrrolidinium Bromide, calculated from its atomic composition: C7H16BrN. It’s not just a trivia fact. In my years of research, the simple step of calculating molecular weight more than once saved colleagues from dosing errors that could throw off results or, at worst, set off a safety issue.
Why Purity Tells More Than Just a Number
Purity often gets ignored until disaster strikes. Purity levels above 98% are considered good for lab work and industrial applications. Any less, and there’s a risk of unexpected byproducts sneaking into your reaction, leading to unreliable data or faulty products. In one of our experiments, a batch with low-grade N-Ethyl-N-Methylpyrrolidinium Bromide forced us to troubleshoot for weeks. Only after spending on characterization tools and outsourcing purity analysis did we spot the issue. This sort of headache can be avoided with a careful check upfront.
Direct Impact on Cost, Safety, and Progress
Buying chemicals with the right specs saves money down the road. Low purity might look cheap, but the hidden costs travel with lost time, damaged equipment, or rejected products. Quality control departments constantly stress testing and documentation. Pharmaceutical researchers take this to heart too, since impurities can interfere with safety profiles for new drug formulations. Regular users should always demand purity certificates and independent analyses when possible. I’ve seen entire product lines recalled just because a vendor slipped on promised chemical quality.
How Purity and Weight Guide Everyday Decisions in the Lab
I’ve worked in labs where weighing out the correct mass based on accurate molecular weight makes a world of difference. Even students learning the ropes gain good habits if instructors emphasize the right figures and checking labels. You see frustration on faces when a compound doesn’t dissolve or react as expected, usually traced back to an unverified bottle of reagent. It’s easy to blame bad technique, but double-checking molecular details should always be the first step.
Smart Purchasing and Handling
Researchers can do better by working closely with reputable chemical suppliers. Ask for NMR, FTIR, or HPLC certificates to back up purity claims. Look up the supplier’s reputation in the market. If budgets run tight, at least conduct basic quality checks in-house. Some universities share equipment for this reason. In industry, solid documentation and random spot checks keep both workers and customers safe. Knowledgeable buyers and users won’t just trust a label; they’ll have proof.
Practical Steps for Better Outcomes
People in the field benefit from making these checks routine. Setting up quality protocols pays off in fewer failed experiments and complaints. Training new lab members to look for both molecular weight and purity shapes responsible habits. I’ve learned to keep analysis reports on file and encourage colleagues to do the same. These small choices keep workflows safer and productivity higher in settings where accuracy matters most.