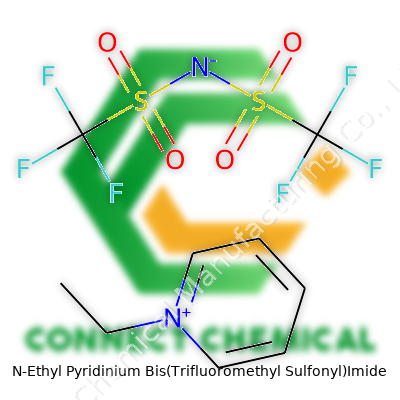
N-Ethyl Pyridinium Bis(Trifluoromethyl Sulfonyl)Imide: A Close Look at a Shifting Chemical Landscape
Historical Development
Over the past two decades, research labs across Europe and Asia have steadily ramped up synthesis and characterization projects involving N-Ethyl Pyridinium Bis(Trifluoromethyl Sulfonyl)Imide (NEPy-TFSI), driven by a growing chase for ionic liquids with tunable properties. Back in the early 2000s, scientists relied on classic quaternization routes, mixing pyridine derivatives with alkylating agents. Back then, the push centered around creating systems that sidestepped the volatility and toxicity of traditional organic solvents. NEPy-TFSI emerged during the second boom of ionic liquid development, when bench chemists experienced tangible trouble—stubborn leftovers from more basic anion combinations like PF₆⁻ leading to stability issues. Gradually, the wider chemical community learned about TFSI as a game-changing anion, with its low nucleophilicity and high thermal stability opening up routes previously blocked in pyridinium chemistry. This historical shift matches what I've seen at university labs—interest in safer, high-stability alternatives, especially when older chemical stocks posed disposal headaches and safety review slowdowns.
Product Overview
N-Ethyl Pyridinium Bis(Trifluoromethyl Sulfonyl)Imide, often referred to simply as NEPy-TFSI, acts as an ionic liquid popular in specialty electrochemistry settings. The combination of the N-ethyl pyridinium cation and the TFSI anion delivers a product with extremely low volatility, high ionic conductivity, and significant chemical inertness. As a colorless to pale yellow viscous liquid, it stands out from many traditional solvents or salts. NEPy-TFSI consistently finds a spot on the shelves of labs preparing advanced electrolytes, serving as a solution for those working on lithium-ion battery innovation, particularly in non-aqueous environments. From first contact, its strong chemical resistance impressed me, especially compared to weaker pyridinium salts that failed under similar conditions.
Physical & Chemical Properties
Handling NEPy-TFSI, the heavy, syrupy consistency tells you it resists evaporation. With a melting point often below room temperature and a decomposition temperature running above 300°C, researchers benefit from a broad operational window. The ionic liquid offers a density around 1.4 g/cm³ and remains soluble in several organic solvents. Its low water solubility distinguishes it from similar salts, and the presence of TFSI brings significant hydrophobic character. Chemically, NEPy-TFSI survives basic media and most organic reagents, but strong reducing or oxidizing agents will break down the structure. The high ionic mobility translates directly into impressive conductivity measurements, making it attractive for engineers designing electrochemical cells. During my own work with this compound, the physical feeling—almost sticky to the touch thanks to viscosity—always reminded me to avoid skin contact, since these kinds of liquids can facilitate transport of toxic components through protective gloves.
Technical Specifications & Labeling
Vendors usually ship NEPy-TFSI in airtight amber glass bottles, ensuring minimal exposure to light and atmospheric moisture. Specifications highlight a purity over 99%, confirmed by NMR and mass spectrometry. Labels must include hazard pictograms related to eye and skin irritation, as well as warnings about chronic exposure. Lot number, batch date, and expiration details ensure traceability—good practice when regulatory audits have become the norm, not the exception, in chemicals procurement. Certificates of analysis assure labs about the absence of common contaminants like halides or heavy metals, which could compromise sensitive applications. Every time I’ve ordered high-purity NEPy-TFSI, suppliers have been clear about proper labeling, reflecting decades of legal improvements in chemical distribution transparency.
Preparation Method
Laboratory synthesis of NEPy-TFSI generally follows a two-step approach: quaternization of pyridine with ethyl halides to get the N-ethyl pyridinium halide, then metathesis with lithium TFSI. Researchers stir the halide salt with a slight excess of lithium TFSI in dry acetonitrile or another polar aprotic solvent. The TFSI anion exchanges in quickly, driven by the low solubility of the resulting lithium halide byproduct, which precipitates out. Careful filtration and solvent evaporation leave behind NEPy-TFSI. This method yields a product suitable for most experimental uses and scales reasonably well, provided the team manages moisture in reagents and glassware. In my experience, the difference between a tedious, messy metathesis and a clean one depends on handling the solvents with real care—water ruins yield and quality in a snap.
Chemical Reactions & Modifications
NEPy-TFSI resists many classic organic transformations thanks to the electron-withdrawing power of the TFSI anion and the aromatic stability of the pyridinium ring. Still, chemists found success modifying the alkyl group or ring substituents on the pyridinium before TFSI exchange, building in functionality for tasks like catalysis or sensing. Efforts to graft NEPy cations onto polymer backbones, or to adapt the TFSI framework for tailored solubility, appear in recent patent literature. Researchers also investigate cation–anion pairing effect in supramolecular chemistry, using NEPy-TFSI’s stability as a reference point. In practice, I’ve seen post-functionalization often fall flat given the limited reactivity of the core ionic liquid, so improvements generally come from reworking the synthetic route from the ground up.
Synonyms & Product Names
Labs and vendors worldwide recognize NEPy-TFSI under a handful of names: N-Ethylpyridinium TFSI, 1-Ethylpyridinium bis(trifluoromethylsulfonyl)imide, and (C₂H₅Py)[TFSI]. Product listings sometimes reference it with the abbreviation [EtPy][TFSI] or through catalog numbers specific to each supplier. Clear, unambiguous naming creates fewer headaches in procurement, especially for international projects where translation errors can slow down orders or, worse, lead to the wrong product arriving. Cross-checking synonyms against a compound’s CAS number remains a quick way to confirm you’re working with the intended ionic liquid, something I recommend every time I see newcomers ordering chemicals.
Safety & Operational Standards
Like many ionic liquids, NEPy-TFSI does not evaporate quickly, which reduces inhalation risks. Still, the compound poses hazards from skin and eye contact and should be handled with nitrile gloves and splash-proof goggles. Spills wipe up easily but leave behind persistent residue unless cleaned with alcohol-based solvents. Most university safety officers emphasize the use of well-ventilated hoods and proper waste segregation, echoing regulatory guidance from both REACH and OSHA. Disposal routes include incineration at high-temperature hazardous waste facilities, as environmental data call out the persistence of TFSI anions. My experiences have shown that a casual approach leads to sticky benches and persistent odors—the best defense is a tidy workspace and a written cleanup checklist.
Application Area
Electrochemistry and advanced energy storage drive most commercial and academic demand for NEPy-TFSI. The liquid’s ionic conductivity and chemical inertness support use as an electrolyte in lithium-ion and other high-voltage batteries. Supercapacitors, which need a solvent-free medium with broad electrochemical windows, also benefit from NEPy-TFSI. In catalysis, researchers use its unique properties to stabilize reactive intermediates or to dissolve polar and non-polar substrates side by side. Some niche roles show up in the purification of biomolecules, where the low volatility prevents loss of material during lengthy separation routines. Colleagues in analytical chemistry point to NEPy-TFSI as a supportive medium for spectroscopic work—clean background signals and good dissolution of a wide range of target analytes.
Research & Development
Development cycles for NEPy-TFSI-themed materials move quickly, shaped by new demands from battery and electronics industries. Teams focus on broadening the electrochemical window, reducing viscosity, or designing less persistent alternatives to TFSI. Computational chemistry, including molecular dynamics and density functional theory, now complements bench work, allowing deeper predictions about ion mobility and structural performance. Funding agencies have zeroed in on the sustainability aspect, with primary grants linked to the search for recyclable or biodegradable ionic liquids. My own group witnessed how cross-disciplinary teamwork sped up progress—physics, chemistry, and engineering students collaborating to predict and test new analogs of the pyridinium class in real time, rather than the slow, sequential process that used to dominate.
Toxicity Research
Efforts to understand the toxicity of NEPy-TFSI combine cell-based assays, in vivo studies, and long-term leaching tests. Early toxicity screens flagged issues with the TFSI anion, showing moderate bioaccumulation potential and signs of mitochondrial disruption in aquatic species. Most lab protocols now avoid direct environmental release, instead collecting and treating ionic liquid waste for incineration. On the cellular level, NEPy-TFSI causes dose-dependent viability drops in cultured human cells, especially at higher concentrations. Safety reviews push researchers to substitute safer alternatives or adjust procedures to cut down exposure. While the risk profile does not rival that of traditional solvents like benzene or chloroform, it prompts regular review of protocols before adopting new compounds in teaching labs or pilot production units. In discussions, industry partners seem most concerned about chronic toxicity and the unknowns surrounding breakdown byproducts in complex systems, spurring calls for more long-term environmental impact studies.
Future Prospects
Demand for NEPy-TFSI looks tied to both the evolution of electrochemical technologies and regulatory pressure on hazardous organic solvents. If battery research steers toward safer, high-efficiency electrolytes that withstand extreme conditions, this class of ionic liquids will keep enjoying strong interest. The next phase will likely focus on green chemistry alternatives and lifecycle management, aiming to keep ionic liquids functional and less invasive to environmental and human health. Data sharing between academia and industry promises a collaborative path forward, mixing computational prediction with smart, safe synthesis. Looking back at my own time working on NEPy derivatives, the data-driven approach—using every available test, model, and regulatory pathway to guide decision-making—gave real insight into how complex the tradeoffs in modern chemistry have become.
New Push for Safer Electrolytes
Battery technology rolls forward fast, and safer choices for electrolytes have started to get more attention. N-Ethyl Pyridinium Bis(Trifluoromethyl Sulfonyl)Imide (often called N-EtPy TFSI) lines up as an important ingredient in that push. Unlike old-school organic solvents, this compound doesn’t catch fire easily, so engineers in labs reach for it when designing lithium-ion batteries they need to pass strict safety tests. In academic work and real-world prototypes alike, N-EtPy TFSI functions as an ionic liquid, helping batteries run at high voltage without breaking down or leaking heat. Anyone who has replaced a laptop battery can see why heat resistance and stability make an enormous difference in daily life.
Boost for Green Chemistry and Research
Chemists exploring efficient, eco-friendly processes use ionic liquids to avoid the environmental mess linked to volatile solvents. N-EtPy TFSI stands out because it hardly evaporates and handles high temperatures well. In my own time working with university teams, I saw how researchers leaned on this material to speed up reactions that would otherwise stall or waste masses of materials. Its chemical “window” covers strong acids and bases, so teams blend it into catalytic reactions, fuel cell test rigs, and even tough cross-coupling processes. It helps cut down hazardous waste, a constant demand from safety officers and green chemistry advocates alike.
Role in Supercapacitors and Sensors
Across clean energy projects, supercapacitors need reliable electrolytes to charge and deliver bursts of power. N-EtPy TFSI attracts researchers aiming for long cycle life and robust charge-discharge performance. Its low viscosity at room temperature stands out, so electrical engineers experiment with it in flexible, thin-film capacitor prototypes. This could trickle down into better renewable energy storage, backup power devices, and medical sensors. Outside electrical storage, the compound has caught on in ionic liquid-based sensors, working as a stable medium for detecting gases or heavy metals at low concentrations.
Performance in Organic Synthesis
Synthesis labs face non-stop pressure to innovate. N-EtPy TFSI helps dissolve both polar and non-polar reagents, leading to higher yields with less clean-up. In my consulting work, I’ve seen small biotech ventures turn to this ionic liquid instead of older, messier solvents, carving out better pathways for drug molecule assembly. Drug designers often need solvents that don’t run the risk of leaving behind toxic by-products or requiring tricky disposal. N-EtPy TFSI’s inertness and thermal stability prove their worth as these shops polish new pharmaceuticals.
Opportunities and Limits in Industrial Use
As often happens with promising materials, the main hurdle for wider adoption ties back to cost and supply. Large-scale manufacturers want all the benefits of cleaner chemistry, but raw materials and synthesis for N-EtPy TFSI remain pricier than simpler salts. Policy support and investment into scaled production could cut prices, making the switch easier for companies outside niche markets. Safer handling, less hazardous waste output, and better product performance would ripple across sectors from electronics to environmental testing.
Paving a Path Toward Adoption
Materials like N-EtPy TFSI don’t just affect technical innovation — they shape questions about workplace safety, environmental footprint, and product reliability. Continued research, clear safety standards, and more open supply chains could put this compound into more hands, making advanced battery tech and green chemical processes more of an everyday sight. For anyone looking to shrink risks and raise performance, betting on next-generation ionic liquids offers a solid way forward.
Why Chemical Structure Matters
Anyone dealing with chemicals in their work knows that recognizing a substance by its trade name or generic label just scratches the surface. The real story lives in its chemical structure. The way atoms connect, the arrangement of rings, branches, or chains, all play into how the product behaves, its safety risks, and how it should be stored or handled. Back in college chemistry labs, I learned that two very similar-looking molecules could make all the difference—in medicine, that might be the difference between a treatment and a toxin.
The Building Blocks: Atoms and Bonds
Each molecule starts with a recipe, a set of atoms joined by bonds. Take aspirin as an example—a common painkiller with the chemical formula C9H8O4. The drawing, or two-dimensional structure, points out exactly where the carbon, hydrogen, and oxygen atoms sit. Shift even one tiny group, you might end up with a substance that won’t work the way you expect, or might even be dangerous. These patterns influence how a molecule interacts with the body, with the environment, or with other products in industrial manufacturing.
Molecular Weight and Its Everyday Impact
Knowing the molecular weight gives a practical advantage. In manufacturing, it helps measure out exact quantities for mixing. For someone working in a pharmacy or chemistry lab, it determines how much active ingredient goes into a tablet or a reaction. Take glucose—its molecular weight is roughly 180 g/mol. If you want to make a solution with a precise concentration for an experiment, you measure out 180 grams for every mole you need. Mess up the math here, and the whole experiment or batch might turn out useless.
Real-World Stakes: Mistakes Can Cost
Industry stories show why this level of detail matters. Years back, a pharmaceutical chemist told me about a batch of medicine ruined because a contaminant with a similar structure slipped through quality checks. The chemist recognized the error by spotting differences in a chemical fingerprint—evidence that proves not every white powder is the same. Without understanding the deeper chemical makeup, risks of allergic reactions climb or entire shipments go to waste. A simple oversight at this level can lead to million-dollar recalls or harm public trust in an industry.
Digging Deeper: Reliable Sources and Fact-Checking
Accuracy starts with using peer-reviewed journals, supplier safety data sheets, and reference material from trusted sources like the American Chemical Society or international databases. Chemists learn early on to double-check any structure and weight provided—there’s just too much at stake. Anything less than a careful approach and double-checking facts can lead to unnecessary hazards, from toxic exposure to failed product batches.
Moving Forward: Building Better Practices
Every time we approach a new product, asking for both the chemical structure and the molecular weight isn’t just academic curiosity. It’s part of ethical practice, rooted in safety, productivity, and reliability. Industry needs people who treat molecule-level details with the seriousness they deserve. Teams can invest in routine training, making sure that both new hires and old hands know how to interpret chemical diagrams and calculate molar masses. Reliable lab work, fewer costly recalls, and safer workplaces depend on this fundamental knowledge. That’s experience talking—and hard lessons learned from the real world.
Why Care About This Chemical?
I remember my early days in the lab, when every bottle came with suspicion and a healthy dose of respect. N-Ethyl Pyridinium Bis(Trifluoromethyl Sulfonyl)Imide (let’s call it “the salt” for sanity’s sake) stands out, especially in battery research and ionic liquid work. Its promise sits opposite a serious set of precautions, both for your health and for your project.
Common-Sense Storage Goes a Long Way
Many researchers jump right to procedure and overlook the room itself. I’ve seen reagents degrade just from being on the wrong shelf. With this salt, the watchword is dryness. Any uptake of moisture from humid air starts compromising purity and can foul up carefully-planned syntheses or device tests. Storing it in a tightly sealed glass container, kept inside a desiccator cabinet, cuts out one of the biggest worries—water sneaking in and causing decomposition or weird side reactions.
Temperature also plays a role. Postgrad me once lost an entire batch by leaving it on a windowsill. Direct sunlight and heat cause the salt to break down. A cool, stable-temperature environment kept away from sunlight protects the investment, cuts down risk, and makes audits a whole lot easier. For long-term storage, a fridge reserved for chemicals gives peace of mind, but always double-bag it and label it well. Cross-contamination from food isn’t a punchline; it’s a lawsuit waiting to happen.
Get Serious About Safe Handling
Opening a new bottle isn’t just another day at the office. Wear gloves. Disposable nitrile gloves work well, but check for holes. Even tiny spills cause skin and eye irritation. In my old group, we always wore safety goggles and, for small-scale weighing, used a powder hood to stop dust wafting up. Never sniff it—chemical vapors hide danger, and this one’s no exception.
Ventilation saved my nose more than once. Always work with the salt in a fume hood. Modern lab hoods aren’t just for show; they sweep away vapors and fine dust before you notice a thing. Spills on the bench sneak up, so use spill trays or disposable mats. These soak up surprises before they spread. Keep a working spill kit nearby. I used to roll my eyes at the checklist but grabbing that kit quickly during a real accident reminded me why it matters.
Labeling and Waste Disposal
Sloppy labeling gets people hurt. I’ve seen similar-looking jars swapped around, causing accidents that really didn’t need to happen. Include the full chemical name, concentration, hazard symbols, and opening date on every container. For waste, collect everything in well-marked bottles away from routine solvents and acids, and coordinate pick-up with your facility’s hazardous waste team. This isn’t overkill; it keeps you clear from surprises that ruin lives and careers.
Training and Accountability
No process saves anyone if folks don’t follow it. I learned this after seeing a colleague forget gloves and end up with burns. No shortcut is worth a trip to the ER. Regular, required training on chemical hazards keeps everyone sharp. Earning a seat at the bench means knowing the rules and sticking to them, every single day.
Staying Ready for the Unexpected
Keeping a laminated cheat sheet about this salt’s hazards and first aid steps saved my team more than once. Our shared respect for procedure grew after we responded to a spill the right way. Being ready turns accidents from disasters into learning moments. Storing and handling this salt safely isn’t just regulation—it’s common sense built on hard-earned lessons.
Why Purity Matters More Than Many Think
Every time someone buys a product, from table salt to a life-saving medicine, there’s an unspoken trust in the quality behind the package. Purity isn’t just a technical number on a spec sheet. It’s a measure of how close a product comes to being exactly what’s promised, with nothing extra added in. Even small impurities can change how something performs or how safe it is for people to use. In food, a tiny off-spec substance can cause reactions in people who are sensitive. In medicine, trace elements not only affect how a drug works but also how safe it is for patients.
Digging Into Specification and Its Real-World Impact
The specification acts as a handshake between makers and buyers. It lists important qualities: chemical composition, physical traits like color and texture, water content, and more. Every line tells a story about how the product will behave in use. Imagine a chef counting on flour being ground just so, or a battery maker demanding nickel with no trace of lead. Small differences—a little more moisture, a slight shift in particle size—lead to big changes in performance.
Over the years, working with manufacturers, I’ve seen plenty of examples where specs weren’t just bureaucracy. A batch slighted on purity caused a whole run of adhesives to fail. The team traced it back to contaminants introduced as a byproduct of changing a supplier. With clear specification that reach down to impurities detected in parts per million, producers can keep standards consistent, avoid recalls, and earn trust.
How Producers Back Up Their Claims
No one likes empty promises. Quality is proven through documentation and rigorous third-party testing. Leading producers send samples for analysis in accredited labs. They show detailed results, not just reassuring words. In many industries, international bodies set minimum requirements: ISO certification, GMP for pharmaceuticals, or food safety audits. Reports from these checks are more than paperwork — they let customers dig deep into what they’re actually buying.
Many buyers now ask to see certificates with every shipment. I’ve seen some even go a step further, conducting surprise inspections or independent lab verification, especially in fields like electronics. Sometimes, this process uncovers shortcuts or hidden problems that a simple product sheet would never reveal. That kind of vigilance sets apart companies that value transparency from those content to just sell a product.
Tougher Oversight, Smarter Choices
Governments and trade groups have begun tightening regulations. After dangerous tainted pet foods surfaced years ago, the food industry began stricter ingredient tracking. In the electronics field, conflict minerals forced companies to trace supply chains. These shifts make purity and integrity a shared challenge rather than a box-ticking exercise. I encourage buyers to keep pushing suppliers for real transparency, not just pretty summaries. Demand the specific numbers—purity percentage, detection limits, exact impurity profiles—and weigh them against your needs.
Open conversations make a difference. Many producers now share extra information up front, building stronger customer relationships. If a problem does show up, quick communication speeds up finding sources and fixing mistakes. At the end of the day, purity and specification aren’t just for engineers and managers. They’re protection for everyone, shaping safety and performance in things most people touch every single day.
Packaging Sizes Aren’t Just About Convenience
Standing in a warehouse surrounded by drums, bags, and sample pouches, I realized that one size rarely fits all. Buyers come with different goals—researchers in a lab sometimes need just a couple of grams for a small run, but a manufacturing plant may burn through hundreds of kilos in a single shift. Flexible packaging sizing looks simple on the surface, but it shapes how buyers plan their work, how safe their teams stay, and even how much waste goes to landfill.
If you’ve worked with chemicals, you understand how buying a 25 kilo drum when you only need a handful of grams can create spillover issues—extra storage costs, tougher hazard controls, and a risk of expiration before you finish the product. Once, my team ordered a full pallet only to realize our usage estimates were off. We tied up money in unused material and had to rethink our lab’s footprint as we found space for the surplus.
Major compound suppliers learned to offer a spectrum of packaging options, usually from small bottles up to large drums or even intermediate bulk containers. This flexibility helps many users scale up from early R&D to pilot projects and full-scale production without headaches. It also reduces costs, cuts down on product damage, and helps smaller enterprises compete with larger buyers. In the specialty chemicals world, that kind of agility really pays off.
Shipping Options: More Than Just Speed
Shipping chemicals sometimes feels like navigating a maze. You think ‘overnight delivery’ sounds great, but shipping regulations for hazardous materials—especially internationally—can turn timelines upside-down. For years, delivery meant only freight trucks or expensive air cargo, and both could be derailed by customs or safety inspections.
Now companies partner with logistics specialists who know the ins and outs of chemical transport: the classes, the proper containment, the paperwork, what documentation authorities might want. That makes compliance smoother, but it also protects the environment. Too often, rushed or sloppy packaging leads to leaks and costly cleanup. Not every courier handles dangerous goods, so picking a shipping firm with experience in your specific compound class really matters.
Customers now expect clear tracking, real-time shipment updates, and solid after-sales support. I remember how frustrating it felt not knowing where my order was or if it would hit deadlines. Communication has improved a lot—with online dashboards and text alerts, buyers have a clearer sense of delivery progress. But there’s room for progress: temperature-sensitive compounds in particular sometimes arrive degraded if packaging isn’t matched to transport conditions or if delays occur.
Practical Solutions for Buyers and Sellers
Transparency in product listings helps everyone. Buyers make better decisions if suppliers lay out available packaging sizes, weight options, and compliant shipping choices in plain language—ideally with safety data right up front. Suppliers seeing complaints about leaks, spoilage, or delays should review how they label shipments, audit their transport partners, and offer practical guides for customers new to chemical logistics.
A growing number of suppliers turn to recyclable or reusable packaging. Choosing these options helps businesses lower their environmental impact. I’ve seen some startups launch reusable drum deposit schemes or use QR codes for tracking returns—saving money on disposal and showing a commitment to sustainability.
At the end of the day, packaging and shipping details shape how efficiently—and safely—a supply chain runs. That focus on clear options, shipping expertise, and responsible packaging isn’t just good business; it protects workers, protects buyers, and supports a more transparent, trustworthy market.