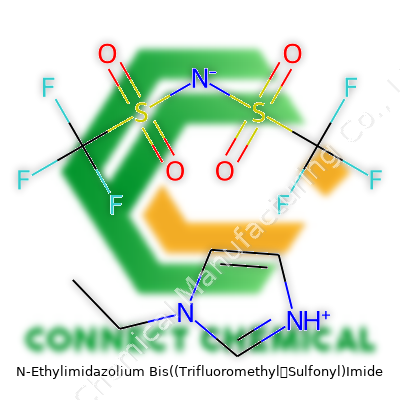
N-Ethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide: A Deep Dive
Historical Development
N-Ethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide stepped onto the scene alongside the wider interest in ionic liquids during the early 1990s. Chemists and engineers searching for green alternatives to volatile organic solvents stumbled across families of imidazolium salts like this one. Over the years, its low vapor pressure and thermal stability began to attract significant research funding. Early academic reports revealed its impressive ionic conductivity, and industrial labs picked up on the chance to shape new electrolytes and solvent systems. The rise of clean tech drew attention to ionic liquids with minimal environmental emissions, leading to larger-scale synthesis in Europe and East Asia. Today, this compound sits in catalogs and warehouses, a product of decades of chemistry advances and a reflection of society’s push for safer, cleaner lab work.
Product Overview
N-Ethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide belongs to a group known for combining strong chemical performance with manageable physical handling. As a room-temperature ionic liquid, its liquid form under normal conditions opens the door to uses in batteries and synthesis reactions. Researchers sometimes refer to it as [C2MIM][TFSI] or just N-EtIm-TFSI, a nod to its complicated chemist naming roots. Major suppliers list the product in high-purity grades, targeting academic and industrial applications where contaminants can derail the chemistry. The cost stays relatively high due to the complexity of its preparation and the growing market for high-tech electrolytes and advanced material processing.
Physical & Chemical Properties
Anyone familiar with ionic liquids gets used to a slippery, viscous liquid with very little odor and a surprising weight in the hand. N-Ethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide crystallizes easily if temperatures drop, but stays stable at room temperature, thanks to its strong ionic lattice. Its density hovers around 1.4 g/cm³, putting it right at the heavier side of most solvents. Thermal stability stretches beyond 300°C, with decomposition producing mainly imidazole derivatives and fluorinated sulfones. This compound resists hydrolysis and oxidation under storage conditions, rarely turning yellow or cloudy like some older generation ionic liquids. Its conductivity makes it valuable for use in electrochemical devices, outperforming many common organic solvents.
Technical Specifications & Labeling
Makers offer this compound in both analytical and industrial grades, with labeling that highlights purity, water content, and key safety warnings. Typical purity specifications exceed 99%, with water content below 500 ppm—key for applications in energy storage where even small impurities cause big problems. Suppliers provide certificates of analysis, and bottles carry UN classification for transport as a non-flammable, non-corrosive substance. Batches ship in amber glass or high-density polyethylene containers to guard against light and accidental contamination. Regulatory data includes REACH registration and guidance for Responsible Care standards, with disposal instructions aligned with both local and international guidelines.
Preparation Method
The process that leads to N-Ethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide starts with alkylation of imidazole, usually with an ethyl halide in a polar aprotic solvent. Operators use carefully dried glassware and inert atmosphere to block any water from spoiling the yield. After the N-ethylimidazolium halide salt forms, the next step introduces lithium bis(trifluoromethylsulfonyl)imide in aqueous exchange, producing a dense, two-phase mixture. The less dense ionic liquid phase separates, washed repeatedly with deionized water to shed lingering ions. Final purification strips the last traces of volatiles under reduced pressure, monitored by NMR or FTIR to verify full conversion. Industrial plants scale this process using continuous flow reactors and membrane-based separation for larger batches, slashing waste compared to older, batch-based approaches.
Chemical Reactions & Modifications
As part of the versatile ionic liquid family, N-Ethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide handles a broad palette of chemical reactions. Its imidazolium cation structure tolerates alkylation, acylation, and ring substitutions at the C4 or C5 position under specific conditions. Chemists modify the ethyl side chain to tune viscosity and solubility, giving different versions for unique engineering tasks. In practice, this ionic liquid dissolves catalysts and substrates for reactions ranging from transition metal-catalyzed coupling to fluorination of organic compounds. Unlike many other solvents, it survives repeated heating and strong base or acid contact, but strong nucleophiles can still attack the imidazolium ring, limiting its use in some situations. These characteristics allow researchers to adjust its properties for selectivity in synthesis or to extend battery lifetime in electrochemical setups.
Synonyms & Product Names
Chemists and suppliers call this ionic liquid by several names: N-Ethylimidazolium TFSI, [C2MIM][NTf2], or just Ethylimidazolium bis(trifluoromethanesulfonyl)imide. Some research papers refer to its shorthand as EMI-TFSI, using the earliest abbreviation conventions. Global catalog numbers and CAS codes ensure easy tracking, since regional regulations demand clarity on chemical procurement. The variety of names comes from the evolving field of ionic liquids, where different research groups coined naming systems before international standards locked in. To avoid confusion in lab ordering, most professionals cross-check synonyms and registry numbers when requesting supplies.
Safety & Operational Standards
Working with N-Ethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide does not call for elaborate personal protective equipment, but gloves, splash goggles, and lab coats remain mandatory. Lab teams know that even stable ionic liquids can irritate skin and eyes on prolonged contact. Careless handling around open flames or strong oxidizers increases risks, as decomposition can produce toxic gases. Proper ventilation and sealed containers help prevent incidental exposure, and safety data sheets stress immediate rinsing in case of accidental spills. Training on transport and emergency handling is standard in both academic and commercial operations. Down the waste stream, operators send spent material to licensed incinerators, obeying strict international protocols to avoid fluorine and imidazolium breakdown products entering the water supply.
Application Area
N-Ethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide stretches across energy, catalysis, analytical chemistry, and materials engineering. Battery engineers value its ionic conductivity and wide electrochemical stability, especially for next-generation lithium-ion and redox flow cells. Catalyst developers pick it for its ability to dissolve both metals and organic ligands, jumpstarting reactions that tend to stall in traditional solvents. In separation science, it works as a mobile phase modifier, boosting sensitivity for tough-to-detect analytes in liquid chromatography. Polymer scientists use it to dissolve stubborn copolymers and create new films with unique heat and electric profiles. Outside the laboratory, specialty manufacturers test this compound in anti-static coatings, sensors, and electrochromic devices. Its resilience under harsh conditions opens pathways for safer, more efficient processes in both small-scale R&D and commercial-scale production.
Research & Development
Labs around the world push into new ground with N-Ethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide, trying to extend battery life, cut toxic emissions, or boost the reach of catalytic cycles. Academic studies probe structure-property relationships, measuring how changes to the cation or anion flip its conductivity or viscosity. Pilot plants experiment with in-line recycling techniques, shrinking environmental impact while lowering costs. Some groups work to blend this ionic liquid with solid-state materials, opening up safer, non-combustible energy storage. Others hunt for ways to upgrade its environmental footprint, such as biodegradable or less persistent derivatives. The push to match lab innovation with large-scale deployment keeps the pace brisk, with regulatory bodies under pressure to quickly evaluate and endorse newer chemical classes while ensuring workplace safety and community protection.
Toxicity Research
Questions about the safety of N-Ethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide create steady demand for toxicological data. Lab testing in rodents and aquatic organisms tracks its acute and chronic toxicity. So far, the results point to relatively low oral and dermal toxicity but reveal that breakdown by-products, such as fluorinated sulfonates, persist in the environment longer than traditional hydrocarbon solvents. Repeated high-dose exposure tends to inflame sensitive tissues and may stress aquatic life when large amounts enter waterways. Industrial hygiene guidelines recommend handling as a substance of low volatility but treating it with respect, restricting entry to the general environment. Research continues to build detailed understanding of the compound’s long-term safety, especially as more applications lead to larger-scale production and use. Regulators and manufacturers both know the need for transparent risk assessment, clear safety communication, and proactive monitoring to head off public health concerns.
Future Prospects
Interest in N-Ethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide is only going up as industries push for greener, safer, and more energy-efficient alternatives in specialty applications. Investment in clean tech drives its adoption in mega-batteries and chemical recycling, while universities turn out graduates fluent in ionic liquids and their quirks. Opportunities continue to expand in data storage, flexible electronics, and modular manufacturing, feeding demand for stable, high-performance solvents. Persistent questions about lifecycle impacts put pressure on chemists to design next-generation compounds with faster breakdown and even lower environmental persistence. Recycling and closed-loop processing will take an ever-larger role, as regulatory standards tighten and consumer awareness grows. The trajectory ahead leans strongly toward integrated, systems-level solutions where N-Ethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide serves as both a bridge and a proving ground for chemical technologies that serve 21st-century needs.
Why Scientists and Engineers Reach for This Salt
N-Ethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide, with its chemical mouthful of a name, solves a range of old and new problems in labs and on production floors. This substance stands out in my experience for its stability and stubborn refusal to evaporate or break apart, even under tough conditions. These qualities lead chemists and engineers to look for jobs it can do that regular stuff simply cannot handle. Its role in advanced batteries, green chemistry, and complex separations gives it influence that pops up in daily tech, often without anyone noticing.
Redefining Electrolytes in Batteries
The shift to renewable energy demands batteries that can store more power, last longer, and resist catching fire. I’ve seen N-Ethylimidazolium-based salts get picked for these jobs thanks to their ability to form ionic liquids. These liquids make safer electrolytes that refuse to leak or burn. Labs testing lithium or sodium batteries use this salt to keep things stable as power cycles in and out across months and years. The data often shows improved life and charge speeds, which offers hope for grid storage and electric cars to catch up with consumer expectations.
Simplifying Industrial Chemical Reactions
Traditional solvents like toluene or chloroform cause headaches when cleaning up after a reaction. Besides being tough on the environment, they often force a tough trade-off between safety and yield. Specialists choose this ionic compound because it stays in liquid form across a wide temperature range and easily dissolves a host of other molecules, especially organic ones. It opens opportunities to streamline catalysis and extractions, allowing more product at lower cost, while reducing hazardous waste. There’s less demand for endless distillation steps or harsh neutralization at the tail end of a process, which saves energy, cuts risk, and frees up resources for other projects.
Stepping Into Electrochemical and Sensor Technology
This material finds its way into the guts of modern sensors and electrochemical devices. Scientists point to its reliable conductivity and chemical stubbornness as reasons for choosing it when building sensors for industrial fumes, lab analyzers, and test strips. These sensors benefit from resistance to oxidation and breakdown, two enemies that sideline typical electrolytes. In practice, this means devices survive longer and deliver accurate results, helping workers hit safety and quality goals. Hard data shows improved detection limits, especially for acidic or reactive compounds that chew through standard materials.
Greener Approaches to Environmental Problems
From oil spills to contaminated soil, cleanup teams seek new answers that cause less stress downstream. Ionic liquids with stable anions and cations like these bring strong separation power to environmental extraction, pulling out tough pollutants. They attract attention because recovery and reuse come easier, trimming costs and waste output. Real-world projects prove these salts help close loops, making reclamation of heavy metals or organic toxics less damaging.
Pushing Toward Safer, Leaner Chemistry
This compound’s impact grows as companies and researchers push to shrink hazardous byproducts and find replacement for volatile organic chemicals. Since its ionic structure stands up to voltage swings and doesn’t build up in air or water, regulators give cautious approval. Added up, these features help tilt the chemistry world toward safer, more practical choices, tucked behind the scenes but driving lasting change in batteries, sensors, and green tech.
The Real World Stakes of Chemical Stability
In the lab or on an industrial scale, chemical stability shapes the daily routine and long-term outcomes. Unstable compounds sometimes act like gremlins, waiting for the slightest shift in temperature or exposure to light before they degrade or react. Every chemist remembers opening a bottle of what should have been a standard reagent, only to find an odd color or unexpected precipitate. That moment often means wasted resources, extra paperwork, and a scramble to ensure safety.
A good example comes from stories of sodium metal. If left exposed, even just for an hour in humid air, a silvery solid turns crusty and dangerous. Water in the air triggers rapid oxidation, and the once reliable material may put people and research in harm's way. Not every compound turns hazardous so quickly, but each has its triggers and breaking points.
Why Environment Matters
Many compounds lose their punch or become risky if handled without respect for their sensitivities. Heat speeds up unwanted reactions for a wide range of chemicals, even those considered shelf-stable under normal conditions. Take pharmaceuticals: the difference between a stable tablet and one that fails quality tests often comes down to whether it sat too long in a warm warehouse or got exposed to sunlight during shipping.
On a personal note, I learned the value of dry, cool storage after losing a batch of reagents during a summer blackout. Fuses melted, fridges warmed, and a four-figure investment in temperature-sensitive materials ended up as hazardous waste. Lesson learned: if the power grid or air conditioning can't be trusted, invest in backup systems—generators, temperature alarms, and silica gel packets for moisture protection.
Labels, Containers, and the Human Factor
Clear, honest labeling often saves the day. Too many accidents start with unmarked or mislabeled containers. I once worked with a team that standardized color-coded labels for every class of chemical—peroxides got red, moisture-sensitive compounds got blue. It took extra time at first, but it cut confusion by half and made audits quicker.
Container material makes all the difference. Volatile chemicals demand glass with airtight stoppers. Plastics can leach or react; some polymers even melt when exposed to strong solvents. Storing acids or bases in metal never ends well, so everyone kept a checklist near storage shelves.
Smart Strategies for Longevity
Shortcuts in storage rarely pay off. Small investments in proper shelving, spill containment, and fire extinguishers seem like overkill until they prevent disaster. Chemical stability is often a slow war of attrition with air, moisture, and time. Even routine updates of safety data sheets can reveal new storage findings. During my graduate training, I caught a new warning in an updated SDS that saved us from incompatible storage of two similar-looking compounds that, under heat, could ruin equipment or worse.
Regular training works better than any written protocol. Employees who update each other on new discoveries or issues with stock keep the whole operation safer and the chemicals steadier. A well-cared-for store of compounds doesn't just protect investments. It keeps everyone breathing easier—literally and figuratively.
Small Habits, Big Safety
Every safe lab or warehouse starts with a culture that values diligence and transparency. It grows with smart purchasing of containers and monitoring devices and thrives when people know why their routines matter. Chemical stability isn't a distant, technical concept reserved for specialists. It’s part of the daily rhythm of anyone who cares about safe, effective science, from student labs to production lines. Putting in the work upfront keeps mistakes rare and solutions simple.
Respecting Labels and Warnings
Years ago, I treated bottles of cleaning supplies like they were just another household item. I barely glanced at labels, let alone read the fine print. After dealing with a mild chemical burn from a splash of bleach, that changed pretty quickly. Labels carry hard-earned knowledge—everything from storage tips to those skull-and-crossbones hazard signs.
Product warnings exist for a reason. Look for hazard pictograms, bold text, and instructions about ventilation or skin contact. The glove icon doesn’t mean fashion advice; it’s a signal your skin shouldn’t meet what’s inside the bottle or bag.
Good Habits: From the Warehouse to the Home
Take a walk through most garages or sheds. You’ll spot open containers and faded safety data sheets stuffed under other junk. Some folks still leave paint thinners or pesticides on low shelves. It doesn’t take much for a pet or a child to reach them. Safe storage means finding a locked cabinet, away from food and high enough so wandering hands or paws stay out.
Transportation calls for its own care. Even a short drive can turn into a disaster if containers tip and chemicals leak. Secure them upright in a vehicle, don’t toss them into the trunk or backseat. I learned that lesson after a bottle of pool acid rolled open on a bumpy road and left burns in upholstery.
Personal Protective Equipment: More Than an Afterthought
Lab coats, rubber gloves, safety goggles—they might look over-the-top for a simple cleaning task, but they shield skin and eyes from unexpected splashes. Some bathroom cleaners can blind in a single accident. So if the label recommends gear, use it. At home, I keep reusable gloves and a pair of clear glasses handy under the kitchen sink, just in case.
Breathing matters too. Strong fumes from solvents or ammonia-based cleaners do more than sting the nose. Over time, they can do permanent harm to lungs. Cracking windows and turning on fans helps, but sometimes a proper respirator does better, especially when the job moves past a light scrub.
Mixing and Measuring: No Room for Guesswork
I used to think mixing a bit of this and that would make cleaning faster. Bleach and ammonia promised a stronger result. The coughing fit that followed punched home why mixing products without checking compatibility is a bad plan. Some combinations release toxic gases, enough to send people to the ER.
Always follow recommended concentrations when diluting chemicals. Overdosing doesn’t speed things up; it only increases risk. Measure with the right tools, not handfuls or cereal spoons. Anything coming in contact with chemicals, even water for dilution, should stay separate from kitchenware.
Clean-Up and Disposal: Staying Safe After the Job
Once the work wraps up, rinsing skin and tools gets overlooked. Soap and water do more than remove residue—they stop the transfer of something hazardous to food or faces later on. Store personal protective equipment away from clothing and wash up thoroughly.
Disposal carries its own traps. Pouring leftover products down the drain pollutes water and pipes. Most towns hold hazardous waste collection days; a phone call to the local recycling center can point you in the right direction. At home, I keep a bin for old paints, solvents, and batteries that never goes out with regular garbage.
Education Goes Hand-in-Hand with Safety
A bit of time spent reading labels or asking questions pays back for years. Every chemical—whether it’s a cleaning product or fertilizer—deserves the same respect as a power tool. No one wants to remember a safety rule because of an accident. Learning from the start helps keep every family and coworker out of harm’s way.
Why Purity Matters
Out in the world, purity isn’t just a science word. In real terms, the grade of a product can make or break a project. I’ve spent enough time tinkering with different materials—whether it’s cleaning chemicals, lab reagents, or building supplies—to see what happens when the expected quality doesn’t line up with what’s inside the package. A construction project feels the pain of weak cement; a researcher hits roadblocks with low-grade solvents. Purity isn’t just about fancy numbers on a bottle. It brings real consequences in reliability, safety, and performance.
Choosing the Right Grade for the Job
Most products on the shelf come in more than one grade or purity level. The food industry has rules about what counts as “food grade,” so nobody wants their flour or salt laced with anything extra. Medical labs bank on the consistency found in pharmaceutical and analytical grades, paying higher prices for cleaner, controlled substances. Getting the wrong grade can waste money, time, and may even hurt someone. It’s not always obvious with a quick glance at the label, though. In my own experience, confusing “technical” with “reagent” grade meant an experiment simply fizzled out—turns out trace contaminants pack a serious punch, especially on sensitive work.
Risks behind Low-Grade Materials
Cutting corners on purity looks cost-effective in the short run, but the results often hit later. Equipment may corrode faster, and the shelf life of formulations can nosedive. Any business that’s had to toss out a bad lot or redo a batch knows the lost hours and wasted resources. The pharmaceutical sector learned this lesson years ago, tightening up controls after too many incidents with impurities. Research by the U.S. Pharmacopeia shows that better standards have reduced risks—adulterated products sparked dozens of recalls before the focus shifted. It’s a reminder: shortcuts with raw materials usually backfire.
Transparency and Testing: Pillars of Trust
Every time I buy something I’ll rely on, I dig through technical datasheets or certificates of analysis. More companies are offering transparency, publishing detailed breakdowns and revealing what’s really inside. This kind of open reporting builds trust. No one wants to glance at mysterious codes; end users need real data to make informed choices. The American Chemical Society has long pushed for clear labeling and honest reporting. Feedback from users helps as well. Highlighting issues with one batch or praising a reliable supplier shapes the future landscape and helps improve reliability.
Building Smarter Standards
Solutions start by insisting on plain language across industries, so buyers know exactly what they’re getting. It pays off for suppliers that take time to educate customers, offering workshops or guides about different uses and grades. Real-world training helps teams recognize warning signs and verify specs before accepting shipments. Certification programs, third-party audits, and stronger regulatory oversight all play a role. Simply put, demanding better standards creates a race to the top.
The Practical Edge
Over the years, I’ve learned not to compromise on grade, even if the price looks tempting. Asking questions, insisting on transparency, and checking certificates builds a safer, smarter workspace. The pursuit of higher purity pays for itself over time, not just in reliability but in the confidence it gives everyone from lab techs to process engineers. Knowing the grade—and understanding why it matters—turns every purchase from a gamble into a smart investment.
Why Shelf Life Makes a Difference
I learned early in my career that the actual shelf life of a specialty chemical isn’t just some number printed on a data sheet. In labs and manufacturing, handling ionic liquids such as N-Ethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide means thinking about how stability influences safety, process consistency, and budgets. Mistakes in storing or assuming a chemical remains sound over the years can upend entire runs and cost companies more than the material itself.
Understanding the Clock
N-Ethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide, known to some researchers as [EMIM][TFSI], falls into the family of imidazolium-based ionic liquids. On paper, many manufacturers estimate two to five years as the shelf life, provided storage tempers extremes and keeps out moisture. Pure, high-quality samples sealed tightly in amber bottles typically look and perform the same, even after a couple of years tucked away in chemical storage. Yet, things aren’t always that simple outside a perfect environment.
Humidity attacks the ions. Oxygen in the air can degrade both the imidazolium ring and the anion. In practice, I’ve opened containers accidentally left unsealed, just to find clumped crystals or yellowed liquid where clear, free-flowing samples once stood. I’ve spoken with colleagues who swear by double-wrapping bottles and adding extra desiccants near leaky fridges. Even with these efforts, lot-to-lot results float apart if the material is old or not managed with discipline. Official paperwork doesn’t always tell the whole story—but pure product, consistently capped, and stored between 2–8°C, avoids many headaches.
What Goes Wrong in Poor Storage
A big misconception is that all ionic liquids shrug off water. I’ve watched students scoop out degraded EMIM-TFSI, blaming their mistakes on poor purification, when the real culprit lurks: water absorbed from the air. Trace moisture triggers hydrolysis. The color shifts. Physical properties slide; conductivity measurements drift. In electrochemical cells and batteries, even small shifts in composition can derail an experiment or erase a competitive advantage. If purity falters, results become unreliable or inconsistent. As someone who once lost a week’s worth of electrochromic device data because a bottle sat out overnight, care with shelf life feels personal and practical.
Making Chemicals Last
Relying on manufacturer recommendations does help. Fresh manufacturing dates give confidence if supply chains run smoothly, but the real solution lies in better local storage and handling. Sealed glass bottles make a big difference, especially those with Teflon-lined caps. In larger operations, inert-gas blanketing or glovebox transfers keep the material away from air. I keep smaller aliquots for frequent use—the bulk container stays untouched as long as possible, limiting contamination risk.
Labeling containers with “Date Opened” and scheduling periodic checks reduces the chance of unpleasant surprises. Many labs have started routine water-content checks by Karl Fischer titration; others test key physical traits before every major run. Simple habits pay off: keeping samples colder slows degradation, and using desiccators or argon atmosphere genuinely matters.
Practical Thinking Over Theory
N-Ethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide promises long-term stability, but daily routines and small decisions define how much of that promise sticks around in the real world. That’s where experience, not just manufacturer claims, keeps science and industry moving forward.