N-Ethylimidazolium Dihydrogen Phosphate: A Practical Walkthrough
Historical Development
N-Ethylimidazolium dihydrogen phosphate didn’t just pop onto the scene overnight; it’s a product of persistent curiosity and patient tinkering. Researchers took lessons from ionic liquids, realizing that putting an ethyl group on imidazole could give the final salt some unique properties. The field of room temperature ionic liquids received a jolt over the last two decades, mostly because older solvents often catch fire or evaporate into thin air, leaving plenty of pollution behind. Scientists began replacing older, volatile organics with ionic varieties—not for fun, but because new demands in catalysis, synthesis, and electrochemistry called for something different. This compound is part of a trend that stretches back to the 1970s, through blood, sweat, and sometimes, strange looking flasks in cluttered labs.
Product Overview
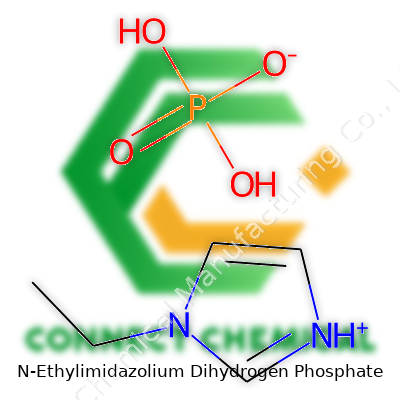
N-Ethylimidazolium dihydrogen phosphate carries a punch: it merges the low-volatility stability of ionic liquids with acidity that lets it slide into various reactions where strong bases can’t tread. Its structure is fairly simple: an imidazole ring with an ethyl tail stuck to one nitrogen, then paired with the dihydrogen phosphate anion. This combo works both as a stable solvent and a mild acidic catalyst. It finds a home on the shelf with other ionic liquids, marked as a clear to pale yellow liquid, resisting easy decomposition or evaporation. Unlike your run-of-the-mill organic liquids, you won’t catch much smell from the bottle.
Physical & Chemical Properties
You can hold a vial of N-ethylimidazolium dihydrogen phosphate without worrying about it spilling everywhere; its viscosity stands a notch above what you get with everyday alcohols or water. The melting point sits below room temperature, setting it firmly in the realm of ionic liquids. It doesn’t ignite easily, and water mixes with it in nearly any proportion. Inside the flask, its thermal stability stretches a bit higher than water, though you shouldn’t expect miracles once you reach above 200°C. Its ionic nature means conductivity stands out, fitting it nicely into the toolkit for green electrochemical synthesis. Chemically, it skirts most strong oxidizers, but keeps its cool around mild bases and acids.
Technical Specifications & Labeling
Bottles arrive with detailed lab stickers. You’ll see its CAS number, purity—often topping 98%—along with warning pictograms since the acidity shouldn’t go ignored. Storage directions ask for a cool, dry spot, away from open flames. The typical label spells out that you’re handling N-ethylimidazolium dihydrogen phosphate, sometimes showing its SMILES strings, and hazards that matter most for folks without a chemical background. You won’t see marketing fluff; instead you get blunt, practical facts so labs or production floors don’t make careless mistakes.
Preparation Method
Making a batch starts with imidazole and ethyl bromide in a straightforward alkylation reaction. The chemist pours imidazole into a flask, stirs in a measured amount of ethyl bromide, and then lets the mix heat gently, making sure the ventilation handles any escaping fumes. The resulting N-ethylimidazole gets neutralized with phosphoric acid, and after some purification steps—typically using vacuum evaporation—the product emerges as a dense, colorless or amber liquid. Mistakes here aren’t trivial; careful attention to temperature and order of addition keeps things safe and avoids unwanted byproducts. If purity matters more, you’ll see extra rounds of washing with organic solvents and drying under high vacuum, ensuring the end product meets those high bar lab specs.
Chemical Reactions & Modifications
People love to tweak N-ethylimidazolium dihydrogen phosphate to fit their needs. Chemists sometimes swap the ethyl for longer or branched groups, learning how this changes melting points or viscosity. Attaching new functional groups fiddles with the compound’s acidity and lets it step into new catalytic roles. You’ll also find projects where the dihydrogen phosphate is replaced by other anions; even subtle modifications here transform the solvent’s surface tension, toxicity, and solubility. These reactions need a measured hand: rushing things or skipping minor purification leads to products that don’t hold up during real experiments.
Synonyms & Product Names
If you search supplier catalogs, you’ll spot N-ethylimidazolium dihydrogen phosphate under a few disguises: N-ethylimidazolium phosphate, [EtIm][H2PO4], or simply the shortened “EIMHP.” On paperwork, one company might misspell the nuance, but the chemical structure draws the same: a five-membered imidazole ring wearing an ethyl suit and paired with the trusty phosphate. Dig around, and sometimes the name turns even longer when listing out entire side-chain variations, but the basic frame remains recognizable. Chemists have a knack for turning standard names into handy labels, so always watch the small print before mixing up your reagents.
Safety & Operational Standards
Handling N-ethylimidazolium dihydrogen phosphate doesn’t bring quite the drama found with strong acids or bases, but respect still matters. Splashes irritate the skin or eyes, and breathing in mist during high-temperature handling invites respiratory discomfort. Chemicals don’t forgive shortcuts, so wearing gloves, goggles, and a proper lab coat prevent most mishaps. Larger manufacturers and labs enforce ventilation rules, insist on spill containment, and check labels before reuse. Waste solutions should head for dedicated waste containers—never straight into the sink. If you’re dealing with kiloliter tanks in a factory versus milliliter samples in a lab, the playbook stays the same: keep an eye on pressure, temperature, and storage, as this keeps all the moving parts safe and legal.
Application Area
This compound carves out a spot in chemical synthesis, especially where traditional solvents fail at bringing together water-averse molecules or where reactions buckle under basic conditions. Solid acid catalysis benefits, since the mild acidity accounts for less side-product headache. It helps in biomass processing, turning renewable feedstocks into platform chemicals through hydrolysis and esterification. Some electrochemical setups choose it for its ionic conductivity and minimal evaporation, letting sensors or battery prototypes operate under greener, less volatile conditions. With its stability, researchers push it into multiple corners of green chemistry, clean energy, and catalysis, while some pharmaceutical labs experiment with its solvent capacity for polar-reactive syntheses.
Research & Development
Ongoing work around the world looks for ways to trim costs, boost yield, and cut down on waste in N-ethylimidazolium dihydrogen phosphate production. Universities and private labs test richer libraries of ionic liquids, comparing how each swaps out cations and anions to drive different reactions. Academic teams keep tabs on environmental fate, mapping how residues break down and what byproducts might emerge under industrial-scale applications. Funding often leans into projects that aim for more recyclable versions or that plug the compound into renewable energy projects, like advanced fuel cells or storage batteries. Research communities talk shop, sharing lessons on scaling syntheses without tripping safety alarms or bleeding resources.
Toxicity Research
No one in the chemistry world ignores toxicity these days. Safety researchers track both acute and chronic effects in workplace and environmental exposures. Single-cell models and higher animal studies show that acute exposure causes at worst moderate irritation. The real concern pops up when residual ionic liquids leak into soil or water. Several studies point out low fish toxicity at concentrations used for lab disposal, but concerns grow if used on an industrial scale. Regulators call for strict disposal protocols, and health monitoring among workers who use the material daily hasn’t flagged persistent harm, but this only applies to sites that stick to best practices. Lab animals that swallow or breathe concentrated fumes show mild inflammation or chemical stress—hardly a reason to panic, but not something to brush aside. Material safety data sheets highlight emergency response actions, favoring a cautious over a careless approach.
Future Prospects
N-Ethylimidazolium dihydrogen phosphate shows real promise in a future driven by green chemistry goals. Its marriage of stability, ionic mobility, and tunable acidity means it’ll keep a foothold in labs and beyond. Large-scale manufacturers already see an edge in switching away from more hazardous solvents, trimming their compliance headaches and lowering insurance bills. What’s missing is a deep-dive into the long-term effects of widespread use, especially in agriculture and pharmaceuticals, where environmental leaks spark the biggest debates. R&D teams continue to chase lower-cost routes for production by tapping renewable feedstocks or using continuous flow reactors, bringing high-purity product to a bigger crowd. Future regulatory changes will likely tighten up safety rules, but clearer standards should help more industries make the leap without hesitation. For now, the path forward combines cautious optimism, sharp-eyed research, and honest dialogue between scientists, regulators, and manufacturers.
Chemistry Pushes Boundaries
N-Ethylimidazolium dihydrogen phosphate—a compound with a name that twists tongues and reminds me how often science trades in complexity—found its way into labs for good reason. It works as an ionic liquid, which means it takes on a liquid state at low temperatures, in contrast to salts like table salt, which melt only at high heat. This trait changes how chemists approach tasks that once demanded harsh solvents and high temperatures.
Cleaner Choices for Industry
Conventional solvents—think acetone, toluene or even water—drive a lot of chemical reactions. These solvents often carry toxic risks or create waste that's tough to manage. N-Ethylimidazolium dihydrogen phosphate, with its blend of an organic cation and an inorganic anion, offers something different. Researchers report that it’s less volatile and less flammable than many old-school options. Several studies tracked its biodegradability and found it stacks up better than most traditional organics. Cleaner processes matter, especially in large-scale pharmaceutical manufacturing or specialty chemical synthesis, where even a small risk scales up fast.
Biomass and Sustainable Chemistry
A hot topic among chemical engineers and green chemists involves converting wood chips, crop stalks, or waste paper to sugars, then to useful fuels or chemicals. This process often starts with breaking down tough plant cell walls made from cellulose and lignin. N-Ethylimidazolium dihydrogen phosphate dissolves cellulose efficiently. Instead of harsh acids or energy-hungry processes, it softens up biomass so enzymes or microbes can unlock the sugars inside. A paper from the journal Green Chemistry in 2020 showed that using this ionic liquid allowed recovery of over 80% of fermentable sugars from agricultural waste—far above what older solvents achieved. Cleaner fuels and bioplastics rest on these small wins.
Enhanced Catalysis and Electrochemistry
In electrochemistry, material scientists work with unusual liquids that let electrons flow but keep unwanted reactions in check. N-Ethylimidazolium dihydrogen phosphate carries charges through a solution while resisting breakdown. This improves energy efficiency and the purity of synthesized products, especially in battery research and advanced coatings. Some electroplating lines looked to this compound to cut down on toxic emissions, benefiting both air quality and worker health.
Safety and the Human Touch
Good chemistry doesn’t rest only on clever molecules—it’s about where those compounds touch workers, communities, or food chains. Ionic liquids like N-Ethylimidazolium dihydrogen phosphate caught flak years back due to uncertainty: does “not volatile” mean harmless, or do risks shift to water systems instead? Recent research started filling those gaps, measuring actual aquatic toxicity and breaking down pathways during wastewater treatment. These studies help regulators and industry decide where this chemical fits—inside labs, pilot plants, or full factories.
Better Ways Forward
Switching to N-Ethylimidazolium dihydrogen phosphate can slash hazardous waste and energy bills in certain processes, but the work doesn’t end there. Industry needs green chemistry to deliver results at massive scale, and that means keeping a close eye on life-cycle impacts. Scientists share what they learn, regulators review the newest data, and the rest of us pay attention to how these choices ripple out—through the workplace, the environment, and downstream to us all.
Why Respect Matters in the Lab
Working with chemicals, even those used every day in research, demands real respect. N-Ethylimidazolium dihydrogen phosphate is no exception. This ionic liquid offers unique benefits for catalysis and green chemistry, but it can bite if treated lightly. Leaks, spills, or sloppy handling invite skin irritation and eye injury. Even experienced folks have felt the sudden sting from careless habits.
No Substitute for Gloves and Goggles
Nobody likes donning more gear than necessary, but barehanded shortcuts in the lab rarely lead to smooth sailing. Nitrile gloves work well; they shield skin from direct contact. I keep a few pairs handy and swap them out if any splash or residue gets on them. Safety goggles turn small dramas into minor annoyances. Nobody plans for a splash in the eye, but a few seconds of safety gear puts that worry to rest. Lab coats not only protect from drips but also stop clothes from becoming an unexpected source of contamination.
Ventilation Keeps Air Clean
Even if something doesn’t smell, vapors and fine droplets can build up in a crowded space. Chemical fumes are tricky – they hang around in the wrong spots, especially when working with tiny quantities day after day. A well-maintained fume hood, checked often for airflow, goes a long way in keeping lungs out of the equation. Breathing easy in the lab beats any shortcut that leads to stuffy rooms and raised eyebrows from health inspectors.
Spill Response Starts with Preparation
Nobody expects spills, but a messy reaction or an elbow in the wrong spot makes them likely over time. Absorbent pads, dedicated waste bins, and quick access to water or spill kits matter most when seconds count. Cleaning up means minimizing contact, scooping rather than wiping, and tossing contaminated materials in a labeled bin. Training sessions every few months keep procedures sharp and coworkers on the same page.
Label Everything, Store with Respect
Proper labeling isn’t just for the neat freaks—it keeps everyone out of harm’s way. Unseen hazards hide in unmarked flasks. Labels with the full chemical name, date, and responsible person cut down on confusion, especially in shared labs. Storage goes far beyond putting bottles away. N-Ethylimidazolium dihydrogen phosphate does best in tightly closed containers, away from acids or bases that could trigger unexpected reactions. Dark, dry shelves offer a safe home. Refrigerators for chemicals should never share space with food or drinks, no matter how limited the space.
Disposal and Environmental Responsibility
Waste can’t simply go down the drain. Ionic liquids, including this one, stick around in water and soil longer than most people realize. Local rules are strict for good reason. Keeping a log of hazardous waste, handing it off to experienced disposal crews, and never mixing incompatible waste make for good habits. It’s not just policy—neighbors and the wider community depend on safe practices.
Building a Culture of Safety
Labs run better when safety is part of the routine, not a box to check for audits. Open communication, regular refreshers, and visible reminders reduce risk. Setting the tone early matters—new team members pick up habits fast. Seasoned researchers can share stories and hard-won lessons, letting the next generation lead with caution and confidence. In my experience, the labs that talk about safety the most often enjoy the fewest accidents.
Understanding the Building Blocks
N-Ethylimidazolium dihydrogen phosphate combines two distinct components: the N-ethylimidazolium cation and the dihydrogen phosphate anion. The chemical formula for the salt comes out as C5H11N2O4P. This reflects the fusion of an imidazolium ring with an ethyl group and a phosphate group carrying two hydrogen atoms.
Curiosity about molecular weight follows naturally. Calculating it involves adding up the atomic weights: carbon (12.01), hydrogen (1.01), nitrogen (14.01), oxygen (16.00), and phosphorus (30.97). Summed up, the molar mass comes to roughly 194.13 grams per mole. While these numbers might look dry at first glance, the reality is, they anchor the behavior and safety profile of the compound.
Why the Details Matter in Real Life
My own foray into chemical research springs to mind here. Plenty of laboratory hours get spent measuring, mixing, and scaling up reactions based on chemical formulas and molecular weights. These aren’t just numbers in textbooks. They shape the amount needed for a reaction, the kind of glassware that’s safe to use, or even which gloves offer the right protection. You can’t fudge the math behind it — a misstep could mean wasted material or, worse, a safety hazard.
The formula and weight also set the foundation for understanding how a compound interacts in solution. N-ethylimidazolium-based salts, for instance, often come up in discussions about ionic liquids. These substances can replace volatile organic solvents in some processes, trimming down environmental impact. Knowing exact weights and formulas lets researchers optimize these alternatives and make them safer for workers and the planet.
Practical Implications in Industry and Research
People working in synthesis rely heavily on these values. Chemical engineers and lab technicians don’t measure their chemicals on guesswork. It’s always about precision. If the molecular weight is off, the error cascades through the experiment or production run. Companies also use these numbers to write safety data sheets and to comply with regulations surrounding safe handling, labeling, and transport. If a label reads “N-Ethylimidazolium dihydrogen phosphate,” accuracy protects everyone down the line — from the person making the batch to whoever disposes of the leftovers.
Approaching Safer, Smarter Chemistry
Chemical data provides a starting point for deeper exploration. With environmental regulations tightening around traditional solvents, compounds like N-ethylimidazolium dihydrogen phosphate play a bigger role. Researchers keep searching for alternatives that do the job without leaving a heavy footprint. Getting formulas and molecular weights right makes that search real — it means safer experiments and, eventually, greener production for everything from cleaning agents to advanced batteries.
Staying grounded in reliable chemical information—proven formulas, true molecular weights—turns the abstract into the practical. Anyone working in a lab, handling chemicals, or designing new materials depends on this accuracy to solve problems, explore potential, and keep both people and the environment out of harm’s way.
Understanding Its Nature
Dealing with chemicals like N-Ethylimidazolium Dihydrogen Phosphate invites a real responsibility. This ionic liquid brings unique features compared to more familiar organic solvents, and those same characteristics shape how we ought to handle and store it in the lab or workplace. Anyone working in a lab with ionic liquids learns quickly that treating them like any old bottle of solvent leads to trouble. One spill or exposure can set projects back and trigger safety concerns nobody wants.
Respect the Material
No fancy storage method matters if the material sits forgotten on a shelf exposed to the wrong conditions. What stands out about N-Ethylimidazolium Dihydrogen Phosphate is its sensitivity. Moisture will change its properties. Heat decreases its stability, possibly leading to decomposition or pressure build-up in closed containers. Direct sunlight may start reactions you don't want. Trust me, storing it “out of the way” in a window-lit storeroom or near hot pipes never ends well.
What works best: a tightly-sealed glass container kept in a cool, dry spot out of the sun. Polyethylene or polypropylene bottles also resist its ionic nature. No leaky corks, no makeshift lids, no “temporary” arrangements that slowly cause air or water seepage over time.
Label and Separate: No Exceptions
Labels running low on detail present risk, especially during a busy day. Clear wording, bold hazard symbols, and up-to-date dates prevent confusion. Never place this liquid near acids, bases, or oxidizers — cross-contamination or an unplanned reaction could follow. In my work, I’ve seen carelessness with storage endanger projects, but more importantly, people. A strong chemical management system, where everything has its proper place and purpose, saves time and nerves. It also aligns with strict lab safety audits that major research centers or pharmaceutical plants embrace today.
Protect Yourself and Others
Protective gloves, goggles, and a proper lab coat are not overkill. While not acutely toxic in the same way as some thionyl or cyanide compounds, contact can still irritate skin and eyes. Fumes, while uncommon under normal storage, could develop if improper heating happens nearby. A clean workspace with spill kits, absorbent materials, and access to safety showers helps limit damage if someone makes a mistake.
Storing chemicals away from areas where people eat or take breaks needs no debate. Accidents happen, but setting good habits ensures they do not turn serious. An organized, well-marked chemical corner in a ventilated storage cabinet remains best practice, regardless of workplace size or budget.
Building a Culture of Safety
Good storage habits start with training and stick with regular checks. Audits and routine inspections highlight those overlooked corners where chemicals tend to accumulate and create risks. Too many near-miss incidents stem from staff assuming a bottle or drum “looks fine” without looking closer. Encouraging everyone to double-check and raise storage concerns makes a real difference.
In summary, sound storage for N-Ethylimidazolium Dihydrogen Phosphate means more than following a material safety data sheet. It means acting from experience, keeping things simple and safe, and looking out for everyone who shares the lab or workspace. These details help keep discovery moving and danger out of the picture.
Getting to Know the Chemical
N-Ethylimidazolium dihydrogen phosphate pops up in research labs focused on green chemistry and energy. Anyone working on ionic liquids knows this name. It dissolves a lot of things you throw at it, and scientists see it as a way to cut down on the use of old-school hazardous solvents. Whenever I read about new chemicals like this, my first question is simple: are we trading one headache for another, or are we really bringing in a cleaner solution?
Is It Safe for the Environment?
Let’s start with transparency. You won’t find much public, peer-reviewed data on how this salt behaves in natural settings. The draw for scientists comes from its low vapor pressure, which means it doesn’t easily turn into fumes. In practice, this keeps it indoors—out of the air—and stops it from adding to smog. On paper, that sounds like a win. Still, the real world is more complicated. A liquid hanging around in soil or water could quietly change the game for microorganisms, plants, or bugs. If it lingers, it builds up. Ecotoxicity isn’t a footnote.
Chasing Biodegradability
Plenty of chemical makers say their new mixes break down easily in the environment. The promise matters, especially since the world is full of persistent pollutants that nobody can just “clean up.” I’ve looked at studies involving similar imidazolium salts, and the takeaway isn’t confidence. Many hang around in soil for months, sometimes barely budging even when exposed to light or air. There’s little hard evidence showing that N-ethylimidazolium dihydrogen phosphate fares better. Standard tests—like OECD 301, which challenges a chemical to biodegrade in a few weeks—haven’t shown this salt vanishing fast.
Beyond the Lab: Challenges Moving Forward
We keep hearing that ionic liquids could change manufacturing for the better, but anyone who’s handled chemical waste knows how the details matter. After the solvents serve their purpose, they have to go somewhere. If we don’t fully understand what happens after disposal—landfill, incinerator, or effluent release—calling anything “green” is just wishful thinking. In the absence of strong evidence for fast breakdown, regulators hold off from giving out eco-labels. That’s a lesson regulators learned the hard way after decades of mistakes with chemicals that stuck around too long.
Some Practical Solutions
Companies and universities pushing these new salts need to treat transparency as Job One. That means sharing full life-cycle studies, not just lab victories. I want to see research on local effects in real ecosystems—streams, fields, waste plants—before giving the all-clear. Designing new salts with easier breakdown paths feels possible. Methods like adding natural-friendly structures, or testing microbial communities, aren’t rocket science. I’d also suggest closer tracking in supply chains, so any hazards get spotted early, not late. Waste management should not be an afterthought either, and end-users should get clear training on what safe disposal really means.
Looking for Reliable Progress
Switching out toxic solvents for newer chemicals only counts as progress if the replacements keep ecosystems safe. Regulators, scientists, and industry all have skin in this game. Relying on assumptions ends badly—whether for a local stream or a supply chain. Without hard data on how N-ethylimidazolium dihydrogen phosphate behaves after it leaves the lab, green claims just don’t hold water. Real responsibility means checking every step, right up to the last drop.