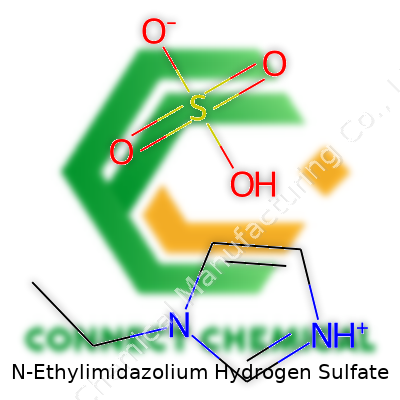
N-Ethylimidazolium Hydrogen Sulfate: A Practical Look at a Modern Ionic Liquid
Historical Development
N-Ethylimidazolium hydrogen sulfate belongs to a class of substances that started gaining attention in the late 20th century. The search for substitutes for volatile organic compounds and traditional acids brought ionic liquids to the table. Chemists found that structures like imidazolium salts could be tuned with different groups and counterions to meet industrial demands. Labs experimenting with green chemistry looked for cleaner solvents and catalysts. That’s when N-ethylimidazolium hydrogen sulfate started turning heads. This compound’s story tracks the wider shift from dangerous, persistent chemicals toward safer choices. Pioneers in synthesis and catalysis kept narrowing in on these ionic liquids, comparing their reactivity and environmental impact to older reagents. By the time ionic liquids caught on, several teams in Europe and Asia had already published research showing how easy it was to swap out alkyl or aryl groups and fine-tune the liquid’s properties.
Product Overview
N-Ethylimidazolium hydrogen sulfate looks like a colorless to pale yellow viscous liquid. It’s known for high thermal stability and very low volatility. It doesn’t burn off in the air or carry a strong odor. Thanks to these features, it shows up in reaction media, electrocatalysis, and even sample preparation for analytical work. Instead of needing extreme conditions or careful containment, workers handle it in standard glassware under fume hoods. This liquid comes in sealed glass bottles or plastic drums for bulk users, fortified to avoid moisture uptake. Researchers and process chemists keep it on hand for its dual ability to act as both a solvent and a catalyst.
Physical & Chemical Properties
Pour some N-ethylimidazolium hydrogen sulfate and you’ll notice its slick, oily consistency. At room temperature, it stays stable, resisting decomposition even when left uncovered for hours. Its melting point sits around room temperature, which helps in handling and storage. Heat it, and it won’t boil until well past 200°C, offering a good window for thermal processes. Despite the ‘hydrogen sulfate’ part, it doesn’t fume or release sulfuric acid vapors. In water, it dissolves with ease, pulling apart into hydrated ions. Conductivity readings show it can shuttle charges effectively, key for applications in batteries or electrochemical devices. Throw it in organic solvents, and sometimes it mixes, sometimes not — depending on the polarity. This feature lets researchers use it both for biphasic and monophasic reactions.
Technical Specifications & Labeling
Most commercial bottles of N-ethylimidazolium hydrogen sulfate bear a purity tag above 98%. That means little room for error in projects needing high selectivity. Labels lay out the molecular formula (C5H9N2 · HSO4), density, water content, and batch number. Safety pictograms stand right next to hazard warnings, usually pointing out skin and eye irritation risks. For supply to regulated labs, a typical product listing includes storage instructions, like “Keep sealed, away from strong bases or oxidizers.” Disposal guidelines and contact details for emergency spill support round out the checklist.
Preparation Method
Making N-ethylimidazolium hydrogen sulfate calls for a simple acid-base reaction. Ethylimidazole joins forces with concentrated sulfuric acid — the process throws off heat and produces the salt right in solution. Stirring at room temperature does most of the work. Once the reaction finishes, workers dry the mix under vacuum or pass it through a rotary evaporator to drive off excess water. Any leftover organics get removed by washing with ether or hexane. The product comes out ready to use, sometimes after filtering through activated charcoal to remove light-hued contaminants. Labs looking for best results use analytical checks like NMR to confirm the final structure.
Chemical Reactions & Modifications
N-Ethylimidazolium hydrogen sulfate handles most acids, bases, and organic reagents without breaking down. Scientists often reach for it in esterification and alkylation reactions, where the ionic nature helps speed up the conversion. The imidazolium ring resists most attacks, so the only common modifications involve swapping the ethyl group for another alkyl. That means the liquid’s core stays intact while side chains get tailored for certain reactions. It can also form mixtures with other ionic liquids, giving process engineers freedom to rebuild their solvent systems for optimal performance. In a synthetic chemistry lab, adding this compound to a reaction mix often reduces the need for extra acid catalysts, which cuts down on dangerous side-products.
Synonyms & Product Names
It goes by several names, but most catalogs list it as N-ethylimidazolium hydrogen sulfate or 1-ethyl-3-imidazolium hydrogen sulfate. International suppliers sometimes use the formula EMIM·HSO4 or simply EMIMHSO4 for shorthand. Some commercial brands tag it as “ethylimidazolium hydrogen sulfate ionic liquid.” Researchers skim the literature for all these versions, especially when tracking down technical notes or toxicity studies.
Safety & Operational Standards
Handling N-ethylimidazolium hydrogen sulfate means following the same rules as with most acid-containing ionic liquids. Gloves, eye protection, and lab coats set the baseline. Skin or eye splashes cause moderate irritation, but the compound doesn’t burn like traditional mineral acids. Clean-up methods focus on dilution and washing, not neutralization with base. Most safety sheets warn against mixing with powerful oxidizers, so keeping these away from the work bench turns into a habit. In scale-up settings, proper ventilation matters more than any fancy engineering controls. Workers rely on standard chemical spill procedures — no special fire protocol or hazard suits needed unless the system involves highly flammable partners. Safety training often points out that, despite its “ionic” label, this liquid doesn’t short out electrical equipment or corrode steel vessels unless left standing for weeks.
Application Area
Industry put N-ethylimidazolium hydrogen sulfate to work in a big way. In green chemistry, the push for less polluting catalysts brought it side by side with palladium or ruthenium — and sometimes even let it replace these metals entirely. Pharmaceutical labs use it in coupling reactions, transesterifications, and oxidation steps, saving on solvents and reducing hazardous waste. In analytical science, adding a bit to complex samples helps draw out stubborn organics during extraction. Fuel cell engineers load it into test cells to improve ionic conductivity and lifespan. In cellulose processing, paper and textile manufacturers tap this liquid for dissolving tough lignin without sharp declines in fiber quality. Battery and capacitor researchers rely on its non-flammability and thermal stability to raise performance benchmarks.
Research & Development
R&D budgets in academia and industry now earmark bigger slices for ionic liquid exploration. Dozens of papers a year cover new ways to tweak the N-ethylimidazolium core, always chasing that “sweet spot” of performance, cost, and green credentials. Teams in Germany, China, and the U.S. published on its recyclability, showing that the compound stands up to multiple reaction cycles with minimal loss in function. Joint projects between universities and chemical suppliers keep pushing for easier, low-waste syntheses with raw materials pulled from biomass — not fossil hydrocarbons. Students still flock to projects on ionic liquids because of the practical payoff in skills and new methods. Conferences feature sessions on large-scale manufacturing and how to deal with leftover or spent ionic liquids, hinting at coming advances in waste recycling and process integration.
Toxicity Research
No chemical comes without risks, and researchers take toxicity screens seriously. Tests on N-ethylimidazolium hydrogen sulfate show modest toxicity compared to old school acids or metal salts, but no one calls it completely harmless. Aquatic toxicity studies raise some flags; spills into waterways threaten fish and small invertebrates. Long-term exposure data in mammals still trickles in, but early findings say that the compound does not build up easily in tissues. Lab test panels focus on skin contact, inhalation, and eye exposure, as well as accidental ingestion. So far, routine handling with basic protective gear protects chemists and factory workers. Rules about storage and disposal aim to prevent release to the environment, with an eye on closed-loop processes in the future.
Future Prospects
N-ethylimidazolium hydrogen sulfate promises more upside as time goes on. The move toward green synthesis draws on its low volatility, non-flammability, and compatibility with a growing list of reagents. Larger-scale plants explore using it for continuous flow chemistry, which drops costs and improves safety. Providers and researchers both see opportunity in custom blends — ionic liquids combined to hit narrow temperature or reactivity targets. Research teams keep filing patents around battery and supercapacitor uses, hinting at real commercial potential in clean energy storage. As more manufacturers look to phase out toxic solvents and heavy-metal catalysts, adoption seems poised to rise. The next decade could bring lower prices, added supply, and smarter recycling systems, pushing N-ethylimidazolium hydrogen sulfate into mainstream chemical production and green tech development.
What Science Does With N-Ethylimidazolium Hydrogen Sulfate
In the world of chemistry, some tools get all the spotlight, but N-ethylimidazolium hydrogen sulfate travels under the radar. Behind long words and little flasks, this liquid gives industries a way around old headaches found in solvent-based processing. Most folks in labs handle it as an ionic liquid, which means it acts both as a solvent and a chemical helper, but it doesn’t evaporate or catch fire the same way many regular solvents do. That cuts down on health risks and improves safety for the people on the frontlines of research and manufacturing.
How It Fixes Problems in Green Chemistry
Industries chasing more responsible chemical routines have to deal with old-fashioned solvents. These solvents break air quality rules and demand pricey ventilation or waste management. N-ethylimidazolium hydrogen sulfate turns this dynamic upside down. Its main use? It cleans up reactions, melts solid materials, and pulls off tricky separations in waste streams—all without polluting the air. Academic labs and chemical plants lean on it to dissolve cellulose, make biodiesel, or refine precious metals from recyclables. People researching batteries or solar cells rely on it to carry out experiments without the risks tied to traditional solvents.
Experience at the Bench
Anyone who has worked in a lab knows that the mess left after a reaction gets expensive fast, in both time and waste disposal. I spent some years cleaning up after traditional reactions, scrubbing glassware loaded with stubborn organic residues. Shifting to ionic liquids like this one cut my cleanup time in half. Toss in less flammable vapors, and spills don’t turn into emergencies. Labmates who deal with big batches always point out the time and money they save. In a facility where cost and safety go hand in hand, these liquids change the day-to-day experience at the bench.
The Industry Snapshot
Paper companies use this liquid to break down tough plant fibers so they can extract cellulose for coatings, textiles, or biofuels. Rare earth industries count on it for separating metals, making recycling electronics less toxic to the environment. The same properties that make it a great solvent in the lab translate straight to full-scale manufacturing, even as factories aim to shrink their carbon footprints.
Tough Questions Still Remain
Every new solution comes with side issues. N-ethylimidazolium hydrogen sulfate looks safer than some options, but disposal stays a concern because these liquids don’t break down quickly outside the lab. Some researchers talk about reusing or recycling the solvent many times, which might cut waste streams dramatically. That’s the direction people in this field seem to be headed: chasing closed-loop systems where solvent doesn’t just get used once, but again and again. So the work continues, both in chemistry and in policy, to make use and disposal stay in step with the greener image these ionic liquids promise. If the world wants cleaner chemistry, these kinds of swaps show the promise—and the problems—to solve next.
What Is N-Ethylimidazolium Hydrogen Sulfate?
N-Ethylimidazolium hydrogen sulfate belongs to a group called ionic liquids, which show up in labs for things like green chemistry, catalysis, and electrochemical applications. This compound’s appeal comes from its low volatility and ability to dissolve many things regular solvents struggle with. Labs worldwide have leaned into its versatility, especially for energy storage and biomass processing. That’s where the spotlight starts shining on safety.
Handling: Why Caution Matters
I once walked into a research group tackling biofuels production, where ionic liquids lined the shelves. Researchers used gloves, worked under fume hoods, and stored chemicals in tightly sealed containers. Even one slip, like forgetting goggles or dismissing the need for a lab coat, could lead to serious trouble. N-Ethylimidazolium hydrogen sulfate isn’t flammable, but don’t let that breed carelessness. This doesn’t take away from other risks.
Health and Exposure Risks
This chemical won’t always offer up an obvious warning like a strong odor or visible fumes. Direct skin contact or inhalation can irritate skin, eyes, and the respiratory tract. Literature shows ionic liquids sometimes linger in the body longer than water-based solvents. Some studies, including one published in the journal Chemosphere, detail how imidazolium-based compounds can cause cytotoxic effects in aquatic and human cells. Not every ionic liquid behaves the same way, and the data on long-term, low-level exposure is still thin.
Environmental Impact
Ionic liquids are often pitched as “green” because they don’t easily evaporate. But “less airborne” isn’t the full story. Waste containing N-Ethylimidazolium hydrogen sulfate shouldn’t go down the drain or into general waste—these substances can stick around in soil and water, harming aquatic life. Germany’s Umweltbundesamt reports the breakdown of similar imidazolium compounds can be slow, which adds another layer of risk to improper disposal practices.
Training and Personal Responsibility
Colleagues new to these chemicals often ask if they’re any safer than older, toxic solvents. One hard lesson from a poorly labeled bottle—a chemical spill, no safety data sheet in sight—echoes in every safety training. Relying on proper labels, clear instructions, and sticking to established hazard communication rules keeps accidents at bay. Rushing or guessing can’t substitute real training. I’ve seen experienced researchers run full chemical risk assessments, double-checking compatibility of every glove and apron before handling. That level of respect for the unknown pays off every time.
Better Handling Practices
The first step is reading the latest Safety Data Sheet (SDS) for this specific compound, not relying on guesses or similar chemicals. Use gloves resistant to corrosive acids, solid eye protection, and good ventilation. Never pipette by mouth. Work only where spills can be contained and cleaned up without spreading residue. Keep waste containers clearly marked and ready for licensed disposal. Regular training updates should never get skipped, even for people who think they’ve seen it all.
Bridging the Information Gaps
Many gaps in safety knowledge come from missing documentation or outdated guidance. Big improvements can come from sharing incident reports, transparent publishing of toxicology studies, and routine audits of lab practices. The real lesson: treat every chemical—new or “green”—with skepticism and respect. This gives everyone a better shot at safety, regardless of lab experience.
Understanding the Basics
Seeing the name N-Ethylimidazolium Hydrogen Sulfate might look intimidating, but it speaks a lot about the compound’s structure and use. If you break it down, it’s made up of two pieces: a cation, which is the N-ethylimidazolium ion, and an anion, which is hydrogen sulfate. The chemical formula for N-Ethylimidazolium Hydrogen Sulfate is C5H9N2+·HSO4−. Converting the cation and anion symbols into a single formula gives you [C5H9N2][HSO4].
Real World Application and Importance
This compound finds itself in the world of ionic liquids, which keep the wheels of green chemistry turning. Ionic liquids don’t evaporate in the air the way most traditional solvents do, and that means people can handle them with less risk of breathing toxic fumes. It also means they cut down on greenhouse gas emissions, which is a real plus for anyone watching Earth’s health. Factoring in that N-Ethylimidazolium Hydrogen Sulfate often acts as a catalyst or solvent in chemical processes, labs looking to switch out harsh chemicals see this as a friendlier alternative.
Across research papers and lab notebooks, scientists have appreciated ionic liquids like this one for their ability to break down tough plant fibers. They play a role in separating cellulose from lignin, which is a step toward making biofuels or bioplastics. This shift toward renewable resources makes a difference in economies that need oil alternatives and tougher sustainability. That’s not just science for its own sake — it can mean new jobs in bio-based industries and new ideas for repurposing plant waste that would otherwise pile up.
What Sets N-Ethylimidazolium Hydrogen Sulfate Apart
N-Ethylimidazolium-based salts don’t act like every ionic liquid out there. The cation’s structure, made with an imidazolium ring and an ethyl group, brings stability to the table and allows for a certain flexibility in the way molecules interact. On the other side, the hydrogen sulfate anion brings acid properties, which is useful when speeding up chemical reactions that need a gentle push. It’s these unique details that make chemists reach for this compound when ordinary acids or water-based solutions fall short.
From my own experience in university labs, choosing the right ionic liquid creates real conversations. Safety comes up every time. Students and professionals alike get behind N-Ethylimidazolium Hydrogen Sulfate because it’s less likely to react unpredictably compared to more volatile substances. Cleaning up spills isn’t stress-free, but the containment is easier, and the lack of fumes brings peace of mind.
Addressing Challenges
Cost, purity, and disposal matter a lot. Ionic liquids weren’t always affordable for small labs or non-academic teams, and even now, tight budgets might mean using older, rougher solvents. Sourcing high-purity N-Ethylimidazolium Hydrogen Sulfate relies on specialized suppliers, so not all schools or companies have easy access. Disposal also deserves attention. Although less volatile, these salts build up in waste, and dealing with them responsibly means more than just tossing them down the drain. Developing recycling systems or recovery processes for spent ionic liquids can ease long-term environmental pressure.
Path Forward
Getting more labs and factories on board with safer ionic liquids like N-Ethylimidazolium Hydrogen Sulfate calls for education and investment. Chemistry educators, industry leaders, and policymakers need to share clear, practical options for sourcing and recycling. Exploring partnerships with chemical suppliers and waste management firms unlocks new solutions. A future where safer, greener solvents become the norm starts with knowing the details — like that simple formula, [C5H9N2][HSO4] — and using it to improve both science and daily life.
Why Take Storage Seriously?
Working in a lab changes the way you look at chemical storage. A friend once stored a similar ionic liquid in a poorly sealed container—pretty soon, a faint rotten-egg odor crept through the storage room. That came from hydrogen sulfide escaping. Even a small slip can set off chain reactions that make people sick or damage equipment. N-Ethylimidazolium Hydrogen Sulfate brings its own set of challenges. Its structure combines an organic cation and an acidic anion, which means it doesn’t always behave like common solvents or salts.
Sensible Storage Starts With Understanding the Risks
N-Ethylimidazolium Hydrogen Sulfate likes to soak up water from the air. This property raises the risk of spills turning sticky and messy, with the added bonus of possible corrosion or toxicity if acids leak onto shelving. Hydrogen sulfate, as an anion, gives the compound strong acidity. Keep it dry and keep lids secure—walking past an open beaker starts to feel like a mistake. OSHA and CDC hazmat guidelines both point out that storing corrosive and hygroscopic materials reduces the risk of irritation, burns, or unpredictable chemical reactions. Protecting lab staff isn’t just about wearing gloves. It involves choosing the right environment from the start.
Direct Steps—No Fancy Strategies
Heavy plastic or glass bottles, capped tight and inspected often, work best. Metal containers end up rusting or pitting thanks to the acid content. Locking a bottle away in the general chemical cabinet seems harmless until you remember that organic liquids or oxidizers may sit right next door. Corrosive storage cabinets offer a better answer. The surface coatings on these shelves resist accidental splashes, and they seal off fumes from escaping into common work areas.
Keep this compound out of direct sunlight and away from heat sources. It breaks down faster and might produce gases that attack seals and labels. Lab temperatures between 15–25°C do the trick. If you work in a climate where rooms get humid, adding a small container of silica gel or another desiccant inside the cabinet fights moisture build-up. Regularly checking the humidity gauge can feel like a hassle, but after one ruined batch or failed reaction, you start to appreciate the routine.
Labeling Reduces Surprises
Poor labeling gets ignored until somebody opens the wrong container in a rush. Write product names, concentrations, hazard codes, and storage dates in clear marker. The time spent marking each bottle and updating lab books beats the confusion and wasted hours that follow a storage mix-up. Sometimes I’ve taped extra sheets with disposal instructions or spill guidance onto the cabinet door—overcautious, maybe, but nobody in the group got hurt.
Disposal, Spills, and Getting Help
Plenty of labs learn the hard way that putting off chemical disposal carries real risks. Any container showing leaks, color changes, or ventilation problems goes straight into a hazardous waste drum. Lab policies should spell out emergency contacts and local regulations. Training sessions on chemical storage sound dry, but chemists who’ve cleaned up a spill know just how quickly mistakes snowball. Working with local emergency teams and reading through material safety data sheets once in a while keeps everyone sharper and safer.
Bringing Home the Message
Storing N-Ethylimidazolium Hydrogen Sulfate means thinking ahead and taking time to check on the little details—tight lids, good labels, dry conditions. Handling chemicals safely never gets old, especially when it keeps people and research on track. Over the years, those habits turn into an unsung foundation for useful, reliable work.
More Than Just a Chemical Name
Some chemicals look complicated on paper, but their uses wind through daily life and work. N-Ethylimidazolium hydrogen sulfate might not turn heads in a conversation, but it’s a member of the ionic liquids family helping change how industries work cleaner and smarter. These ionic liquids mostly stay in liquid form at room temperature and avoid problems common to solvents like evaporation or catching fire. That’s a big deal where safety and cleanliness matter most.
Powering Greener Chemistry in Synthesis
Organic synthesis, that’s the heartbeat of pharma, flavors, and plastics, often relies on solvents that leave a mess behind. Picture a lab or big chemical plant—lots of glassware, warmth, and waste. Traditional solvents can float out into the air, sometimes risking worker health, and eventually building up as hazardous waste. N-Ethylimidazolium hydrogen sulfate offers a cleaner option by keeping reactions rolling along without spewing fumes or breaking down too soon. My experience in a university research lab showed how these ionic liquids cut down on the headache of solvent recovery. Recovery rates went up, waste came down, and the air stayed clearer.
Catalysis Gets a Boost
Anyone working with chemical reactions knows a good catalyst changes the game. You can speed up a reaction, make side products disappear, or even bring tough molecules together. Factories mixing up pharmaceuticals or fine chemicals look for faster, simpler ways to hit their targets. This compound finds use as a catalyst and sometimes a reaction medium, especially in acid-driven steps. It tends to help with selectivity, getting more of what’s needed from each reaction cycle. A few years ago, a colleague in the chemical industry explained how swapping traditional acids for ionic liquids slashed their water use and handling hassles.
Cleaning Up Metal Processing
Refining or recycling metals is gritty, energy-intensive work. Solutions based on N-ethylimidazolium hydrogen sulfate have gained ground in extracting metals like copper or rare earths from ores or scrap. Because these ionic liquids barely vaporize and pull metals out more cleanly than mineral acids, refineries see less equipment wear and dangerous mist in the halls. Fewer process interruptions and less risk underfoot keep union crews and managers both satisfied. In some pilot tests, copper recovery shot up without the sharp drop in air quality that usually tags along.
Fuel Cells and Batteries Eye the Prize
Energy storage stands at a crossroads, and safer, more stable electrolytes are in high demand. Ionic liquids step up here too. N-Ethylimidazolium hydrogen sulfate helps conduct ions between electrodes while refusing to catch fire under tough conditions. This quality turns heads in labs testing next-generation fuel cells or batteries. My time reporting on renewable energy startups showed these researchers keep trying out new combinations, and ionic liquids remain high on their lists for both safety and lasting power.
Future Looks Practical and Safer
Lab workers and plant operators benefit directly from chemicals that keep to themselves instead of floating through the building. With N-ethylimidazolium hydrogen sulfate, industries aiming for cleaner reactions, easier metal recovery, and safer power options pick up real, day-to-day gains. The price point and recycling pathways still pose challenges, but research groups and manufacturers keep pushing for more affordable production and better reuse methods. If industry keeps investing in smarter solvents, the ripple continues—fewer spills, less waste, and healthier workplaces down the line.