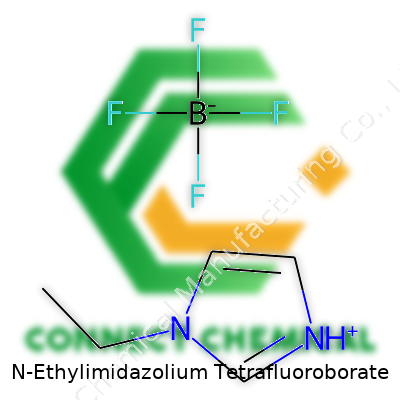
N-Ethylimidazolium Tetrafluoroborate: A Deep Dive into Its Role in Modern Chemistry
Historical Development
Scientists have spent decades exploring ionic liquids. In the 1970s, labs saw growing interest in imidazolium-based salts, not as just another curiosity, but as a step away from volatile organic solvents. N-Ethylimidazolium Tetrafluoroborate soon earned a place in discussions. Researchers noticed its stability, pointing to practical advantages for chemical reactions. Labs gradually recognized the value in handling a salt with low vapor pressure and strong ionic conductivity, giving teams exploring electrochemical processes or green chemistry a better path forward. The shift toward ionic alternatives owes much to early work in this field, showing the world that chemistry could lean away from flammability and harsh fumes. This salt reflects a journey from bench-top curiosity to industrial cornerstone, thanks to patient refinement and clear wins in lab efficiency.
Product Overview
N-Ethylimidazolium Tetrafluoroborate does more than sit on a shelf. Technicians handling electrolytes know it as a reliable ionic liquid, clear or lightly yellow, and always easy to spot once you know what to look for—a crystalline salt with distinct cation and anion structures. With the rise of energy storage and battery research, this compound shifted from niche research to bulk warehousing. Suppliers now deliver it with assurances about purity and batch traceability. Quality matters, especially for applications running on precise redox processes or analytical standards.
Physical & Chemical Properties
This salt melts at room temperature, forming a fluid with low volatility—barely any fumes, unlike chlorinated alternatives. It dissolves well in polar solvents such as water and acetonitrile, and thanks to its ionic nature, it resists common breakdown routes. Chemically, the imidazolium cation carries extra stability from electron delocalization, while the tetrafluoroborate anion shrugs off most aggressive acids and bases. Conductivity numbers catch the eye, especially in devices built for efficiency. Its moisture sensitivity, though, never quite leaves the equation; lab workers invest in dry handling and careful storage. Experience shows that impurities, even in the 0.1% range, can throw off conductivity and reactivity, so instrumentation for purity checking becomes standard equipment.
Technical Specifications & Labeling
Spec sheets tell a detailed story—appearance, melting point, moisture content, cation and anion purity, and absence of byproducts. Labels on factory drums cite accurate batch numbers, origin, and a certificate of analysis, all critical for reproducibility in research and industry. For shipping, hazard codes stand out, highlighting both safe handling and compliance with international guidelines. Every minute spent reading these details often pays off in fewer failed experiments and safer workspaces.
Preparation Method
Manufacturers produce N-Ethylimidazolium Tetrafluoroborate through direct alkylation. The process starts with imidazole and an alkylating agent, bringing in ethyl groups to form N-ethylimidazole under basic conditions. Next comes a reaction with tetrafluoroboric acid, exchanging chloride or bromide anions for tetrafluoroborate. Careful control over temperature, purification by crystallization or extraction, and diligent drying steps make or break the final yield. Chemists who have run large-scale syntheses know how finicky purification can be—trace water or incomplete conversions easily show up in downstream quality tests. Automation and analytical controls stepped up production consistency in recent years, so end users can trust the chemistry behind their supply.
Chemical Reactions & Modifications
Chemists use this ionic liquid in reactions that demand stable, low-volatility media. Electrosynthesis teams favor it for its wide electrochemical window and resistance to side-reactions. Its imidazolium core can anchor further modifications, leading to a library of derivatives. In some reactions, researchers try swapping out the ethyl group or the BF4 anion to tune properties. Beyond being a reaction medium, this compound occasionally enters catalytic systems—helping dissolve or activate catalysts that traditional solvents just cannot manage. The ionic nature often influences selectivity and rates, something every synthetic chemist pays close attention to during optimization.
Synonyms & Product Names
You find this compound listed as 1-Ethyl-3-imidazolium tetrafluoroborate or EMI-BF4. In product catalogs, names shift slightly—sometimes written as Ethylimidazolium tetrafluoroborate, or under systematized codes. In journals, researchers might shorten to “EMIBF4” for brevity. Despite the naming shuffle, the true test comes with CAS numbers and batch certificates—industry insists on exact identifiers to avoid surprises. Cross-checking synonyms matters whenever teams plan new purchases or scale up methods published in the literature.
Safety & Operational Standards
Workers handling N-Ethylimidazolium Tetrafluoroborate take gloves and goggles seriously. Skin contact can cause irritation, and accidental ingestion or inhalation brings real risks. Any lab spill means a cleanup with absorbents, good ventilation, and disposal practices that meet hazardous waste protocols. Written training guides spell out safe storage—dry, sealed containers away from reactive chemicals, with regular inventory checks. Emergency response plans address fire (even if flammability sits low), spills, and first-aid, since preparedness limits harm. Cross-contamination in reactors can change toxicology outcomes, especially in scale-up, so cleanliness and batch separation mean less uncertainty and fewer headaches.
Application Area
This salt earns its keep in electrolytes for batteries and supercapacitors, a favorite for designers working to push energy density and safety beyond what standard solvents allow. Analytical chemists use it as a supporting electrolyte in cyclic voltammetry or electrochemical sensor work, measuring fast redox outcomes without erratic current spikes. Some pharmaceutical research teams lean on ionic liquids for tricky separations or to stabilize air-sensitive catalysts. Environmental engineers test it in pollutant extraction because it dissolves a range of organic compounds. The push for greener solvents ensures demand stays solid—regulatory agencies keep a watchful eye, so compliance never drifts from center stage. In sector after sector, this salt lets researchers sidestep some toxicity and flammability of traditional options, opening doors for novel chemistry.
Research & Development
Investigators keep probing deeper—changing the length of alkyl chains, swapping anions, and loading in transition metals to invent new ionic liquids built around the imidazolium platform. N-Ethylimidazolium Tetrafluoroborate often acts as baseline for benchmarking conductivity, viscosity, and solvent power. Funding agencies monitor this area closely, linking research output to the global push for safer, more sustainable materials. Grant writers reference advances in catalysis, battery life extension, and resource-efficient process chemistry when drawing up new proposals. Educational labs rely on this salt as a hands-on teaching tool for students to explore ionic interactions and practical lab safety at the same time.
Toxicity Research
Academic and industry toxicologists run detailed studies on exposure limits. Most findings agree: this compound poses lower inhalation risks than organic solvents, but it can trigger skin and eye irritation, and under certain breakdown conditions, hazardous byproducts may develop. Research teams review bioaccumulation and aquatic toxicity, as ionic liquids sometimes persist in the environment. Strict protocols in waste management help reduce entry into water streams. Employers track regulations set by authorities such as REACH or OSHA, and ongoing updates shift as more data accumulates. Continuous review remains essential, since next-generation applications depend on clear answers about safety and environmental load.
Future Prospects
What comes next ties directly to the clean energy transition and growth of functional materials. N-Ethylimidazolium Tetrafluoroborate stands ready for broader roles in advanced batteries, fuel cells, and catalysts where safer, more reliable ionic media become a competitive edge. Market reports predict sustained demand as manufacturing scales and regulations grow tighter on traditional solvents. Research trends show collaborations between university chemists and industry partners, looking at both new uses and safe disposal methods to close the lifecycle loop. With each advance in process optimization, this salt finds its way into new sectors, reinforcing its value as a go-to ionic liquid for teams working at the cutting edge of chemical science.
Solvent for Difficult Chemical Tasks
N-Ethylimidazolium tetrafluoroborate pops up in labs dealing with complex chemical reactions, especially in places where water and regular solvents don’t deliver. Its structure gives it that special status: stable under high temperatures, able to handle acids and bases, and non-flammable. Chemists turn to this liquid for its knack for dissolving stubborn salts and organic molecules. Ionic liquids like this one break traditional solvent limits by offering a mix-and-match platform for scientists. Since it doesn’t evaporate easily, lab workers can recover and reuse it, making it a greener choice compared with older chemical standbys.
Electrochemistry and Batteries
Every time you hear about new battery materials, chances are ionic liquids played a background role. N-Ethylimidazolium tetrafluoroborate often lands in electrolytes—the stuff between the battery electrodes—thanks to strong thermal stability and a wide voltage window. Lithium-ion battery developers keep searching for safer, longer-lasting cells, and this compound often becomes their go-to for testing prototypes. It supports high current flow without breaking down or catching fire, pushing boundaries for next-generation energy storage. The same properties make it valuable in supercapacitors, which power up fast and release energy quickly in short bursts.
Role in Green Chemistry Movement
There’s an industry-wide push to lower toxic solvent use in manufacturing. Companies look for chemicals that fit into tighter regulations and reduce environmental impact. N-Ethylimidazolium tetrafluoroborate gets attention because it doesn’t turn to vapor, so workers face less inhalation risk, and it doesn’t spill into the air or ground as easily. Major chemical producers even design synthesis routes to use this ionic liquid as a reaction solvent, cutting down on washing, neutralization, and waste disposal steps. The result: simpler clean-up and less hazardous leftovers.
Organic Synthesis and Catalysis
Building complex organic molecules for medicines or specialty materials often involves tough steps with harsh, old-school chemicals. With this ionic liquid, reactions can speed up and yield more product. It helps shunt electrons or ions in ways that traditional solvents can’t, letting researchers work with trickier ingredients or design smarter catalytic cycles. In some pharmaceutical labs, swapping to N-ethylimidazolium tetrafluoroborate leads to pure product without hours of extra purification. It's not just about academic chemistry; even flavor or fragrance makers chase down purer mixes this way.
Beyond the Lab: Real-World Adoption Barriers and Future Solutions
Despite all this promise, real-world adoption sometimes stalls. Ionic liquids tend to cost more than classic solvents, and many factories balk at new upfront costs for training and equipment adjustments. Some chemists still worry about long-term safety if large spills occur, since the breakdown products remain a bit mysterious. To move past those bumps, manufacturers and researchers can share results openly, especially around long-term toxicity and recyclability. Cheaper production routes—from renewable sources, or using less energy during manufacture—would help. Regulatory clarity helps too: if safety data stays transparent, more companies can weigh risk and reward on solid ground. Experience says that new materials go furthest not just by outperforming the old ones but by making life easier for the folks using them every day.
What Science Tells Us
N-Ethylimidazolium Tetrafluoroborate stands as a precise name within the world of chemical compounds. Its chemical formula is C5H9N2BF4. For those who care about the details, this formula is more than just a string of letters and numbers. It tells you exactly how many atoms of carbon, hydrogen, nitrogen, boron, and fluorine link together to form this substance.
The molecular weight of this compound is roughly 199.95 grams per mole. To most people, that number might sound abstract, but to those mixing chemicals, manufacturing new materials, or running experiments, even a slight deviation from the precise molecular weight could throw off results or damage expensive equipment.
Why This Compound Draws Attention
Ionic liquids, like N-Ethylimidazolium Tetrafluoroborate, caught my attention a few years back while reading about their role in green chemistry. Traditionally, labs relied on volatile organic solvents—many of which are flammable, toxic, or hard to dispose of safely. Ionic liquids offer safer options. This specific compound doesn’t just reduce risk; it lets scientists fine-tune reactions, cut down on waste, and pursue more sustainable paths for both academic and industrial research.
In electrochemistry labs I’ve visited, the switch to ionic liquids made a real difference. We could run experiments at higher temperatures without worrying over lost solvent or nagging safety issues. Researchers using N-Ethylimidazolium Tetrafluoroborate noted better solubility for certain salts and metals, not just marginal gains. This could reshape how batteries are built or how complex organic reactions get scaled up.
Pitfalls and Lessons Learned
Handling any salt containing tetrafluoroborate carries its own share of responsibility. Fluoride-containing compounds demand strict protocols. I remember a sharp chemical smell coming from a waste bottle during one summer research stint. That traced back to incomplete labeling and careless storage of used ionic liquids, including the tetrafluoroborate salts. Exposure to even modest amounts could cause irritation—one lab mate ended up with a nosebleed after ignoring a minor spill on his gloves. Professional oversight cuts those kinds of problems, but shortcuts create hazards any time safety gets skipped.
A key problem surrounds cost and purity. N-Ethylimidazolium Tetrafluoroborate isn’t cheap. If a supplier delivers material with unwanted impurities, research schedules stretch out, budgets balloon, and sometimes, months of results fall apart. Getting reputable suppliers is a lesson everyone picks up quickly, usually after one expensive and frustrating misstep.
Looking for Better Solutions
Chemists keep searching for less-toxic alternatives. Safer disposal paths for ionic liquids stay at the top of research agendas. At conferences and in community meetings, experts swap notes about new recycling methods and greener synthesis steps. Some newer studies look at biodegradable ionic liquids that might sidestep the environmental risk that comes with halogenated compounds.
If the field keeps moving forward, the mix of safety, performance, and sustainability can improve for everyone working with these salts. Understanding the true chemical facts—like the formula and precise molecular weight—lays the groundwork for responsible innovation and improved industrial practices.
Walking Into a Lab, You Notice the Air
It’s one of those things you learn quickly: moisture in the air changes everything. Some chemicals love a humid room, others fall apart or change in ways that scientists spend hours cleaning up later. N-Ethylimidazolium tetrafluoroborate isn’t notorious in textbooks, but it still expects a little respect from users because it doesn't react well with water vapor hanging around. Having spent long days prepping ionic liquids, I always looked out for bottles fogging up or showing signs that the world outside had seeped in.
What Happens to This Salt in Air?
N-Ethylimidazolium tetrafluoroborate is what folks in the lab call “hygroscopic.” This means that it draws water straight from the air into itself. Take off the cap, leave it exposed, and you’ll see clumping or a shift in texture over time. This water uptake weakens its value in syntheses since the end result—your reaction mixture—doesn’t see just the chemical, but an unknown extra amount of water. Accurately weighing out a product turns into a guessing game if the salt has sopped up moisture without anyone noticing.
Hygroscopic Substances Change the Lab Flow
No one likes using impure reagents. Keeping a substance dry may look easy, but soon you realize most rooms, even climate-controlled ones, can’t totally kick humidity out. Working with this salt means scooping quickly, sealing the bottle double-fast, and often putting it in a desiccator. Plastic spoons, dry gloves, and a healthy dose of paranoia go a long way. If a project fails, you start thinking about the little things you missed—like whether water found its way into your ionic liquid. I used to grumble when repeating experiments, only to realize a slow air leak in a supposedly sealed container was enough to throw things off.
Looking at the Science
Researchers have spent time breaking down how ionic liquids like N-Ethylimidazolium tetrafluoroborate react to moisture. Evidence shows these salts take up water via hydrogen bonding, and once enough water gets in, it starts acting less like a pure ionic liquid and more like a strange salt-water slush. This doesn't just affect lab results. Some applications—like batteries—rely on the stable, anhydrous nature of these materials. A tiny mistake leads to lower performance and shorter lifespan.
Solutions You Can Trust
To keep this compound dry, store it tightly sealed, inside a desiccator or under an inert gas like nitrogen. Many suppliers now pack these salts in moisture-resistant bottles with desiccant pouches. Opening and closing containers quickly also helps, as does weighing out portions in a glovebox if the stakes are high. Automated moisture analyzers reveal hidden water, so labs serious about pure results check stocks before use. I’ve found that one overlooked silica gel packet can make all the difference, saving hours and costly chemicals over a year.
No Silver Bullets, Just Consistency
N-Ethylimidazolium tetrafluoroborate isn’t unique for pulling water out of the air. Many ionic liquids play this same trick, though some less than others. Solid preparation, good habits, and a careful approach form the backbone of reliable experiments. The science may get complicated, but the routines don’t: keep the air out, and the chemistry works the way you expect.
Gain Confidence with Safe Storage
Lab work often revolves around a host of compounds—some stable, some finicky. N-Ethylimidazolium tetrafluoroborate falls solidly into the “handle with respect” camp. This isn’t sodium chloride, so tossing a bottle onto a cluttered shelf won’t cut it. Anyone who’s spent long hours working with ionic liquids gets that moisture finds its way into every nook and cranny. This stuff pulls water from the air, and that spells trouble over time. Even if the eye can’t spot a problem, the chemistry won’t lie.
For lasting purity, keep N-Ethylimidazolium tetrafluoroborate in airtight containers. Glass or high-density polyethylene bottles won’t react with the compound, and good lids keep moisture away. I like to use a desiccator for the extra assurance—a habit picked up after years of troubleshooting reaction hiccups and instrument complaints. A desiccator with silica gel or a molecular sieve underneath solves most headache-inducing moisture issues. If possible, avoid wide-mouthed bottles; smaller openings mean less contact with the atmosphere every time you open the lid.
Set the temperature low and stable, away from direct heat or dramatic temperature swings. Many labs store similar compounds between 2°C and 8°C, right alongside sensitive reagents. If a fridge smells like old takeout, clear it out. Good cold storage ensures longer shelf life, especially if you buy in bulk. I’ve seen too many labs lose expensive chemicals to neglectful fridge organization and broken thermostats.
Handling with an Eye on Safety
Handling starts with knowledge. Thumb through the latest SDS before opening the bottle. This chemical doesn’t carry the notorious hazards of strong acids or oxidizers, but don’t let that lull anyone into carelessness. Use gloves—nitrile works well—plus safety goggles and a lab coat. My early years saw a few close calls, and personal protective equipment always made the difference.
Run all transfers and weigh-ins inside a fume hood. Some ionic liquids, especially those with tetrafluoroborate, can release fumes if heated or mixed incorrectly, as detailed in recent studies. That risk grows if you spill on a warm surface or mix with incompatible substances. A hood means any unexpected vapors have somewhere better to go than your lungs. If a spill does occur, clean it up right away with absorbent wipes. Residue attracts water, and the risk of slow degradation creeps in with every missed spot.
Small Habits Help in the Long Run
Label containers right after opening—even here, confusion can do real damage. I once mistook a bottle of N-Ethylimidazolium tetrafluoroborate for a similar salt, setting back a synthesis by weeks. Clear, unambiguous labeling helps everyone in the lab keep mistakes to a minimum. For long-term users, set up a simple inventory and sign-out system. Lost time hunting a misplaced bottle costs more than a few minutes.
For disposal, follow local regulations. Most waste contractors accept ionic liquids, but only if sealed and labeled properly. Never pour into a sink or toss into general trash—recent research on fluorinated byproducts underscores environmental worries.
Experience drives better chemistry. Building solid storage and handling habits doesn’t just save money—it keeps work safer, smoother, and helps everyone rest easy after a tough day at the bench.
Beyond the Labels: Everyday Risks in the Lab
Working with chemicals like N-Ethylimidazolium Tetrafluoroborate brings a constant challenge. Day in and day out, most chemists grow familiar with the smell of solvents and the itch on their skin that comes from rushed glove changes. But this compound, used for ionic liquids and battery electrolytes, raises the stakes. The warning signs aren’t there just for show. Tetrafluoroborate-based salts remain tricky because they sound less threatening than “cyanide” or “hydrofluoric acid,” yet they can bite just as hard in the right circumstances.
Sneaky Hazards: Skin, Eyes, and Air
It takes just one missed step to see what happens. Spilled drops on skin can cause irritation. Eye contact sets off burning and redness – the sort of pain that sticks in your memory. This isn’t the sort of material you want anywhere near a lunch break. Inhaling dust or fumes from N-Ethylimidazolium Tetrafluoroborate kicks off coughing and throat pain, sometimes landing staff in a nurse’s office or sending them home early.
The stuff grows even more troublesome if it comes in contact with water. Tetrafluoroborate ions can break down and release hydrofluoric acid – a notorious chemical with a record for dissolving bone and moving through gloves. The acid acts faster than most realize; the pain can set in too late for easy fixes.
Why Knowledge Beats Luck
Complacency is the real enemy. Many accidents happen with compounds that slip under the radar. I remember a time in grad school when a colleague handled this salt without goggles because he thought it “just smelled funny.” Rest of the week, he worked out of one eye. Most labs keep Material Safety Data Sheets handy, but nobody reads them as often as they should. Industry reports from the American Chemical Society point out that a slight uptick in ionic liquid work has come with a rise in splash injuries and inhalation incidents.
Simple Habits That Save Fingers and Lungs
Putting on good PPE feels like an inconvenience until you realize what’s at stake. I always reach for thick nitrile gloves, full splash goggles, and a snug lab coat before weighing out the white powdery stuff. Swapping gloves after spills and never touching your face becomes second nature over time. For any weighing or transfer, a properly functioning fume hood cuts down risk – the kind with the sash set low. Ordinary face masks can’t block chemical fumes, so those stay in the drawer.
Old glassware sometimes holds residue that reacts with new chemicals. Every piece should get rinsed and dried out before use. Anyone who has watched acid eat through a plastic beaker learns not to reuse without double-checking.
The Bigger Picture: Training, Storage, and Planning
Training remains the biggest shield against disaster. Real walkthroughs beat online slideshows. Safe storage matters, too; sealed original containers in cool, dry cabinets keep this compound stable. No food or drinks belong anywhere near chemical storage. Having a real, practiced response plan for spills means people react fast instead of freezing up.
Labs that build safety into their routines see fewer accidents. People move slower and pay more attention when they know the outcomes. Stopping to check procedure or calling out a possible hazard works better than handling consequences afterward.
In the end, dealing with N-Ethylimidazolium Tetrafluoroborate isn’t just about avoiding fines. It’s about heading home with the same number of working fingers and clear lungs as you started the day with. That’s a win worth every step.