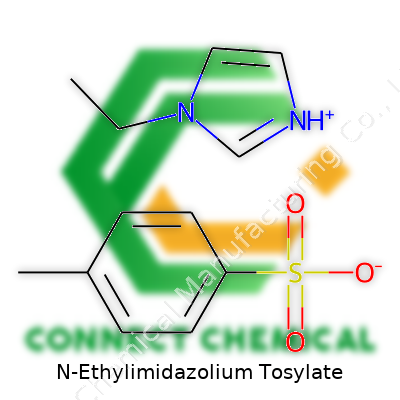
N-Ethylimidazolium Tosylate: Shaping New Horizons in Chemical Innovation
Tracing the Roots: Historical Development
N-Ethylimidazolium Tosylate developed from the expansion of ionic liquid technology. Chemists began realizing the value of imidazolium-based ionic liquids as tunable solvents in the late twentieth century, especially for energy storage and green synthesis. Early literature points to pioneers seeking non-volatile, thermally stable solvents, eventually honing in on the pairing of the N-ethylimidazolium cation with the tosylate anion. This specific combination delivered desirable melting points, stability, and robust performance in reactions once considered difficult with conventional solvents. Such developments drew interest from both academia and industry, eager to reduce hazardous emissions and elevate laboratory safety.
Diving In: Product Overview
N-Ethylimidazolium Tosylate presents itself as a member of the imidazolium ionic liquids family. It consists of an N-ethylimidazolium cation bonded to a tosylate anion. This pairing lends the compound both organic solubility and ionic conductivity. Such features tip the scales for reactions needing stable ionic environments, like organic transformations and electrochemical applications. For chemists and researchers, this compound represents a multi-purpose tool, adaptable within a range of fields, from organic synthesis to energy storage systems.
Attributes: Physical and Chemical Properties
Clear or slightly yellow as a viscous liquid at room temperature, N-Ethylimidazolium Tosylate features melting points often below 80°C, setting it apart from traditional salts. Its moisture tolerance means it can exist stably without significant clumping, maintaining a usable form even after exposure to ambient conditions. Conductivity readings often surpass those of conventional solvents—values reflect both high ionic mobility and a stable lattice. As an ionic liquid, it doesn't emit volatile organic compounds, a quality that reduces inhalation hazards and improves air quality in laboratory settings. With hydrophilic and lipophilic domains, it acts as a versatile medium for both organic and inorganic species.
Tightly Defined: Technical Specifications & Labeling
Industry suppliers generally package N-Ethylimidazolium Tosylate with detailed specifications. Purity levels often extend beyond 99%, with water content under 0.5% w/w to minimize reaction variability. Labels include melting point ranges, shelf life estimations, recommended storage (usually within dry, room-temperature confines), and hazard pictograms relating to irritant potential. Clarity on batch numbers allows for traceability, which proves useful in regulated environments such as pharmaceuticals or electronics manufacturing. Bulk lots come with Certificates of Analysis, covering identification (such as NMR and FTIR spectra), ensuring consistent results across different procurement cycles.
From Lab Bench: Preparation Method
The synthesis of N-Ethylimidazolium Tosylate starts with N-ethylimidazole and p-toluenesulfonic acid, typically reacting in a solvent-free setup to limit waste and contamination. Stirring at ambient or gently elevated temperature allows the acid-base neutralization, forming the imidazolium salt directly. Solvent extraction and repeated washing with non-polar solvents help drive off excess precursors and any colored byproducts. Final drying under reduced pressure brings about a product ready for scale-up or direct use. This route minimizes hazardous waste, echoing the green chemistry values that inspired the compound’s development.
Transformative: Chemical Reactions & Modifications
Beyond serving as a mere solvent, N-Ethylimidazolium Tosylate engages in myriad chemical processes. Its unique cation-anion pairing enhances various catalytic cycles, especially in alkylation, Diels-Alder, and even as an electrolyte in electrochemical cells. Chemists have tinkered with substituents on the imidazolium ring, tweaking chain lengths to adjust solubility, viscosity, and affinity for target molecules. The tosylate anion can sometimes be swapped for other sulfonates, broadening its compatibility in sensitive syntheses. Collectively, such adaptability fuels research into higher-efficiency processes and novel reaction pathways.
Known By Many: Synonyms & Product Names
Suppliers and journals refer to N-Ethylimidazolium Tosylate using a smattering of synonyms: 1-ethyl-3-methylimidazolium tosylate, [C2mim][OTs], and EMIM Tos, among others. These names arise from slight variations in substituents or abbreviation conventions. Catalog numbers often vary between suppliers, making it important for buyers to double-check molecular descriptions rather than relying solely on trade names. Standardized nomenclature in published literature reduces confusion, supporting the reproducibility scientists rely on.
Keeping it Safe: Safety & Operational Standards
N-Ethylimidazolium Tosylate asks for a careful approach in the laboratory. While it lacks the volatility of traditional solvents, accidental skin or eye contact can lead to irritation. Wearing appropriate gloves and safety goggles stays non-negotiable. The compound’s hygroscopic nature means tightly sealed containers save headaches later by avoiding water uptake. Disposal routes involve high-temperature incineration under controlled conditions, as neither municipal waste streams nor standard drains can break down its ionic structure. Facilities using large amounts of this compound benefit from local exhaust ventilation and periodic monitoring for trace contaminants, especially where purity is critical, such as in pharmaceutical development.
Beyond the Beaker: Application Area
Research and industry teams harness N-Ethylimidazolium Tosylate in organic synthesis, electrochemistry, pharmaceuticals, and even as a medium for advanced material fabrication. One standout application involves acting as an electrolyte in lithium batteries—its stability and low volatility offer improved safety margins over conventional electrolytes. In catalysis, the ionic liquid provides a unique environment for transition metal complexes, enhancing yields and selectivities in coupling reactions. Biotechnology labs have started turning to this compound to stabilize enzymes outside their native environments, pushing the boundaries in biocatalysis. Makers of specialty polymers use it to dissolve and process monomers otherwise challenging to control.
Pushing the Limits: Research & Development
Investigations into N-Ethylimidazolium Tosylate often focus on expanding its use as a truly green solvent. Teams have shown interest in recycling ionic liquids across multiple synthetic cycles with minimal performance dropoff, addressing concerns about resource use and waste. Pairing the compound with tailored catalysts enables new transformations, sometimes with dramatic improvements to energy or atom economy. Battery researchers have tuned its composition further, seeking to suppress dendrite formation and support stable cycling even at high rates. In my own work with undergraduate chemistry students, using ionic liquids like this one can dramatically improve safety and reproducibility compared to volatile, flammable alternatives.
Weighing the Risks: Toxicity Research
No compound earns its spot in industry or academia without a thorough look at toxicity. Early research flagged some ionic liquids as persistent or even bioaccumulative, urging caution in their adoption. For N-Ethylimidazolium Tosylate, animal studies suggest moderate irritant properties but little in the way of acute toxicity, provided proper handling. Chronic exposure studies continue as researchers look for subtle effects on aquatic organisms—biodegradability remains a challenge, given the sturdy ionic bonds and structural stability. Regulatory agencies demand comprehensive safety data before approving use in consumer products, ensuring that advances in green chemistry do not trade one hazard for another.
Looking Ahead: Future Prospects
Fields like energy storage, advanced synthesis, and green manufacturing look to N-Ethylimidazolium Tosylate as a source of innovation. Researchers have started building more functionalized imidazolium salts with biodegradable anions, hoping to strike a balance between performance and environmental footprint. Automated synthesis and robotics match well with ionic liquids, reducing time-consuming handling and boosting reproducibility in discovery chemistry. As more labs share their experiences, best practices for reusing, recycling, and safely disposing of these liquids are emerging. In the drive toward sustainability, N-Ethylimidazolium Tosylate stands as a clear candidate for further investment, refining not just how chemists work, but also helping shape how laboratory science manages its own footprint in the long run.
What Stands Out About N-Ethylimidazolium Tosylate?
N-Ethylimidazolium Tosylate is a name that may not come up in everyday conversation, but it matters quite a lot in chemistry circles. The main reason is its unique qualities as an ionic liquid. Ionic liquids have shaped up as problem-solvers across research and industry since their liquid form brings new options to the table. N-Ethylimidazolium Tosylate belongs to a family of salts that stay liquid at room temperature. This sounds small, but in many reactions, going liquid means skipping the harsh solvents that harmed health or the environment. This shift to cleaner chemistry makes it a key piece in the toolkit for sustainable science.
Real-World Uses in Chemistry and Beyond
Lab workers use N-Ethylimidazolium Tosylate to run reactions that need a gentle hand. Lots of organic chemists use this ionic liquid for making special kinds of chemical bonds, especially bonds involving sugar molecules or other complicated compounds. In my experience, working in the lab with traditional solvents like dichloromethane or toluene, you get harsh smells, risky fumes, and messy waste. N-Ethylimidazolium Tosylate swaps out that risky mess for a liquid that handles the chemistry but creates much less danger for people handling it or the soil and water downstream. The safety angle counts a lot if you have ever spent time venting a hood or lugging solvent waste to disposal.
This chemical also finds space in material science. When building polymers or crystals that need careful control of temperature and solvent, N-Ethylimidazolium Tosylate steps in and keeps everything friendly and predictable. People exploring battery technology or alternative energy often favor it to help shuttle ions around or hold pieces together in electrolyte solutions. Energy storage needs solvents that do not break apart easily and will not burn at the smallest spark. Here, this ionic liquid checks both boxes.
Environmental Impact and Why to Pay Attention
Few people worry about obscure ionic liquids, but I have learned from my time in university labs how much even the smallest change in solvent footprint can mean for the environment. Swapping traditional organic solvents for ionic liquids can help cut back on volatile organic compounds, some of the worst contributors to air pollution in chemical manufacturing. The world produces thousands of tons of lab waste every year, much of it from solvents. Go greener, and you can chip away at that environmental cost, even in small batches.
Not everything about ionic liquids, including N-Ethylimidazolium Tosylate, lands as a win. Some break down slowly outside the lab, so waste management needs to keep pace. More research should focus on biodegradability and long-range safety data. I have seen labs focus on recovery and reuse programs where they distill and repurpose ionic liquids, which can lower both cost and environmental stress.
Paths Forward: Safer Science, Smarter Solutions
N-Ethylimidazolium Tosylate gives chemists a practical way to practice cleaner science. As research keeps pressing for greener methods and safer alternatives, this chemical offers a real chance to make progress—one reaction at a time. Teaching more students about ionic liquids, investing in safer waste treatment, and working with manufacturers to rigorously test products could all help expand safe use. As someone who has seen both sides of chemical safety, it’s clear: changes like these matter, and this ionic liquid is one place where the shift has already begun.
Getting to Know N-Ethylimidazolium Tosylate
N-Ethylimidazolium tosylate might sound complicated, but it's really a combination of two distinct chemical pieces that stick together thanks to basic chemistry. The core piece is the N-ethylimidazolium cation. Picture a five-membered ring—three carbons, two nitrogens—with a little ethyl group (two carbons, five hydrogens) dangling off one of those nitrogens. The ring is aromatic and shares electrons around its circle, lending the structure some extra stability, much like how certain neighborhoods get a reputation for resilience because people look after each other. That ethyl arm gives it new properties compared to plain imidazolium, affecting things like melting point and solubility.
On the other side, tosylate comes into play. Chemists often call it “TsO−” or p-toluenesulfonate. That’s a benzene ring, holding a methyl group (just a single carbon and three hydrogens) directly across from a big, acidic sulfonate group (made up of a sulfur atom, three oxygens, and holding a negative charge). The negative charge stabilizes the positive charge on the imidazolium, and the combo melts and dissolves in unique ways, making it more useful in certain lab situations than either half alone.
Why This Combo Matters
I’ve found in the lab that ionic liquids like N-ethylimidazolium tosylate make life easier for a whole range of processes—from green chemistry reactions to solvent systems that just don’t work with water or oil-based options. Their unconventional setups let scientists run reactions without dangerous solvents. That shift shapes safer workplaces and greener supply chains. The structure explains a lot: the bulkiness and charge separation keep these molecules liquid at room temperature, instead of forming neat solid crystals as most salts do.
This structure also fine-tunes the reactivity. The imidazolium core can do more than float around; it eases electron movement around its ring, and that affects catalytic cycles. The tosylate doesn’t just sit back either; its size and shape let it nudge the properties of neighboring molecules in unique ways. For instance, certain catalysts work faster or more selectively because of that bulky tosylate group. In pharmaceutical and materials science work, little changes like this can mean faster production runs or safer end products.
Real-World Impact
It’s not just about what happens on a glass slide under the microscope. N-ethylimidazolium tosylate finds a use in making polymers, processing cellulose, and as a supporting electrolyte in batteries. Companies push for safer, more recyclable solvents, and this compound heads the list of alternatives. I’ve worked with it as part of teams chasing lower waste and fewer greenhouse emissions. Even so, scaling up comes with a price. Ionic liquids can cost more, both in dollars and in energy needed to manufacture them. But the appeal is strong, especially for scientists and engineers solving problems that old-school solvents can't address safely or efficiently.
Path Forward
The solution to practical bottlenecks in using new solvents like N-ethylimidazolium tosylate comes from better manufacturing processes and deeper study into their environmental fate. Researchers track breakdown products and search for greener synthesis paths. Open data sharing between academia and industry reduces duplicate effort and leads to safer designs. Ultimately, understanding these molecular structures unlocks smarter, safer, and more sustainable solutions across chemistry-dependent fields.
The Real World of Lab Chemicals
Anyone who has handled ionic liquids like N-Ethylimidazolium Tosylate knows how fast a good experiment can turn into a headache if storage isn’t managed right. This isn’t just about keeping things tidy—it’s about safety, reliable results, and protecting your budget from waste.
Why Care About Storage?
This compound, favored in organic synthesis and as a catalyst, brings efficiency but also a few quirks. Moisture and air can mess with its purity faster than you expect. I’ve opened more than one bottle of ionic liquid to find a sludgy mess, just because a lid was loose or humidity crept inside. Even indirect air contact will speed up hydrolysis, especially in a damp lab.
What Actually Works?
Not every chemical demands the same level of fuss. For N-Ethylimidazolium Tosylate, keeping it in a tightly sealed glass bottle draws a line between good science and trashing half your stock. I avoid plastic containers whenever possible because some ionic liquids slowly leach, warp, or absorb weird odors if left in most plastics. Clear glass lets you spot cloudiness or unexpected crystallization—signs something changed.
Every time someone asks where to stash this compound, I recommend a dry, dark spot far from water sources and open bench tops. Even though N-Ethylimidazolium Tosylate remains stable at room temperature for short periods, cool (not freezing), stable storage works better. Temperatures around 15-25°C keep most ionic liquids steady. Humidity above 50% shortens shelf life—even with a cap on—so use desiccators or add a few packets of silica gel in storage cabinets for an extra layer of defense.
Labeling and Practical Tips
I label every bottle with the date opened, lot number, and my initials. Skipping this step means no way to track freshness—especially when several researchers share supplies. Once, I traced stubborn product impurities back to old, half-used stock with no label information. Nobody wants that.
Do not transfer to oddly shaped flasks when it’s time to aliquot. I prefer glass vials with screw-on tops so I’m not dipping the same spatula into the full bottle every week. Even a little cross-contamination from a careless day gloves can change your whole batch.
Smart Practices and Safer Workspaces
Keep N-Ethylimidazolium Tosylate away from oxidizing agents and acids. They don’t just degrade the compound—they risk dangerous reactions. I’ve seen the aftermath of careless acid storage. Segregate chemicals thoughtfully, based on reactivity, not just alphabetical order on a shelf.
Unwanted exposure doesn’t just ruin reagents. Breathing in powders or skin contact, even small splashes, matters. Laboratory safety sheets cite mild toxicity, so long sleeves, gloves, and goggles belong in daily practice—not an afterthought for inspections. Prepping your workspace ahead of time means you spend less time responding to accidents and more time getting good data.
Better Results Start with Consistency
Quality science isn’t only about what goes into your flask. A little attention to how you store chemicals like N-Ethylimidazolium Tosylate pays off. It keeps projects on budget, stretches your supply, and protects the integrity of every experiment and every colleague who shares the lab space.
Stepping Into the Lab: What’s in the Bottle?
Every so often, a chemical name pops up that catches everyone’s attention. N-Ethylimidazolium Tosylate sounds like something reserved for the back shelves of university chemistry labs, but its use keeps growing, especially in research around green chemistry and specialty solvents. That growth pushes practical questions to the surface—just how risky is this stuff if you work with it, or if it ever gets out of the lab?
Looking at Real Risks, Not Just Labels
A Material Safety Data Sheet (MSDS) gives a quick feel for how cautious to be, but staring at rows of hazard symbols doesn’t always get to the heart of day-to-day concern. N-Ethylimidazolium Tosylate often gets described as an “ionic liquid.” These materials attract attention for their low volatility and use in novel reactions, but that reputation for being “friendly” or “safe” doesn’t hold up unless we dig into toxicology studies and real-world handling.
Some ionic liquids get credit for being less flammable than old-school organic solvents. Just because fire risk drops, though, doesn't equal a pass for health safety. Inhalation does not seem to be the main worry—these liquids usually don't form much vapor at room temperature. The concerns shift to skin exposure and ingestion. Recent literature shows that certain imidazolium-based salts can cause moderate irritation, and some can make it through the skin more easily than you might expect. Toxicity often depends on which ions get combined—ethylimidazolium adds to human exposure risk, while tosylate brings its own baggage.
Listening to Experience: Lessons from the Field
I’ve worked in labs where even a faint whiff of solvent meant double-checking the ventilation, and where every splash on a glove caused a pause. That caution pays off because long-term exposure stories aren’t always dramatic, but they add up. Chronic low-level contact with organic chemicals—ionic liquids or otherwise—leads to dry skin and unpredictable sensitivities. A colleague with a high tolerance found himself dealing with eczema after handling similar salts for years, even without ever spilling a drop. Reports from industrial settings back this up—repeated skin exposure can set off irritation, and the long-term risks around cellular damage are still being pieced together.
Staying Ahead: Practical Safety Beats Guesswork
Safe handling comes from respecting uncertainty as much as hard data. Gloves, eye protection, and lab coats set a basic standard, but real control means understanding exactly how chemicals interact with protective gear and making sure procedures get followed even on a busy day. Don't just trust your gut—a check of the latest studies helps. Right now, there isn't sweeping evidence that N-Ethylimidazolium Tosylate causes acute health crises at typical lab exposures, but the lack of deep, long-term human data on novel ionic liquids leaves an uncomfortable gap.
Ventilation and regular cleanups, along with minimizing open containers and splashes, remain foundational. Training counts—a quick walk-through on how to handle a spill or dispose of waste beats any poster on the wall. For large-scale users, engineering controls and health surveillance programs aren’t overkill.
Building Toward a Safer Future
Snappy safety slogans won't solve this puzzle. Getting ahead of health hazards takes a mix of skepticism and staying current with research. Companies and academic labs owe it to staff and students to go further than the minimum, pressing chemical suppliers for full toxicity data and collaborating on exposure studies. No need to panic, but underestimating the long-term story of chemicals like N-Ethylimidazolium Tosylate risks more than just irritation. Careful, daily respect for chemicals, old and new, makes science truly sustainable and keeps those stories out of the pages of occupational health reports.
Decoding Its Practical Value
With chemical names like N-Ethylimidazolium Tosylate, most people picture a string of letters and numbers on a label tucked in the back of a research lab. Yet, this ionic liquid has been getting attention for much bigger reasons. Labs and factories aren’t using it just for the name—it gets real jobs done. As someone who has spent time at the intersection of chemical research and industrial R&D, I’ve watched N-Ethylimidazolium Tosylate evolve from a niche lab curiosity into a behind-the-scenes workhorse in modern chemistry.
Making Chemical Reactions Run Smoothly
In synthetic chemistry, keeping reactions tidy gets harder as the molecules grow. Solvents either react when they shouldn’t or refuse to carry heavy molecules. So chemists have started to look beyond the old school choices like water or common organic solvents. N-Ethylimidazolium Tosylate steps up because it stays liquid at room temperature and doesn’t vaporize easily. Research projects in organic synthesis choose this ionic liquid as a solvent or as a catalyst. I’ve seen teams use it to speed up the acylation of amines, a basic reaction with roots in drug manufacturing. It tends to improve yields and sometimes lets researchers cut out extra purification steps.
Opening Doors in Cellulose Processing
Anyone who has ever tried to dissolve or regenerate cellulose knows how stubborn it can be. Most solvents do little. Ionic liquids like N-Ethylimidazolium Tosylate can break down cellulose and lignin, clearing the path for biofuel development or biodegradable plastics. Researchers working on greener processes for textiles often look at N-Ethylimidazolium Tosylate because it outperforms other solvents when dealing with plant-based materials. With more consumer pressure to move away from fossil-derived plastics, better cellulose solvents have impact far beyond the lab.
Advancing Materials Science
Making sure advanced materials like polymer electrolytes work under real-world conditions is no small task. The quest for better batteries and flexible electronics has pushed chemists to rethink the liquids they use. N-Ethylimidazolium Tosylate helps craft ionic conductive gels. I’ve worked with grad students using this compound to fine-tune ionic conductivity and thermal stability for their prototypes. The longer shelf life and reliability of devices built using these gels can’t be ignored.
Improving Catalysis
Catalytic reactions need a medium that won’t gum up the chemistry or break down over time. Traditional organic solvents bring hazards and often call for strict temperature control. Companies searching for safer, more robust alternatives have integrated N-Ethylimidazolium Tosylate into processes that rely on reusable solid catalysts. The ionic liquid remains stable and doesn’t evaporate or poison the catalyst, which means less waste and lower costs over time.
Challenges and Future Directions
Every promising compound rolls out with a list of challenges. Costs keep some companies cautious, especially for large-scale needs. Plus, the environmental impact of production and disposal calls for greener synthesis routes. Some teams are already working toward recovering and recycling the ionic liquid from process streams. Investment in this area could pay off with more sustainable industrial cycles.
As the field of green chemistry keeps growing, practical chemicals with a proven track record stay in demand. N-Ethylimidazolium Tosylate still faces hurdles, but its flexibility and performance give it a spot at the table where innovation meets industry.