N-Ethylimidazolium Trifluoroacetate: A Practical Chemistry Commentary
Historical Development
Chemists began experimenting with imidazolium-based ionic liquids in the late twentieth century, searching for alternatives to volatile organic solvents. The trifluoroacetate anion came into play as the focus shifted toward ionic liquids that offered higher thermal stability, lower toxicity, and improved solubility profiles. The marriage of the ethyl-imidazolium cation with the trifluoroacetate anion resulted in N-Ethylimidazolium Trifluoroacetate. Research papers dating to the early 2000s show steady growth in interest as this compound proved effective for dissolving biopolymers, facilitating homogeneous catalysis, and serving as a medium for various organic reactions. Universities and industrial labs, especially in Europe and East Asia, have helped propel its presence, recognizing that safer, more robust solvents may be the key to modernizing classic chemical processes.
Product Overview
N-Ethylimidazolium Trifluoroacetate stands out among ionic liquids for its ability to interact efficiently with both polar and non-polar substrates. In personal research, its qualities have saved hours of solvent evaporation and cut down the need for repeated extraction steps. Chemists rely on its stability under mild to moderate heating and enjoy the ease with which it can be recycled. This compound often appears as a clear, slightly viscous liquid and, compared to many traditional organic solvents, generates negligible odor. Chemical suppliers are keen to meet rising demand across research and manufacturing, offering packaged volumes that suit small labs as well as bulk users.
Physical and Chemical Properties
N-Ethylimidazolium Trifluoroacetate generally presents itself as a colorless to pale yellow liquid at room temperature, featuring a melting point below ambient conditions. Its density sits higher than water, and the trifluoroacetate anion lends it remarkable chemical resilience. The compound resists hydrolysis and maintains ionic character across a broad water composition spectrum, making it compatible with both aqueous and organic materials. Strong hydrogen bonding capabilities pop up as a distinguishing feature, contributing to its solvency strengths for complex biopolymers like cellulose and chitin. Thermal decomposition occurs at elevated temperatures, where it releases trifluoroacetic acid vapors—a point worth remembering in scale-up environments.
Technical Specifications & Labeling
Every bottle of N-Ethylimidazolium Trifluoroacetate shipped to the bench comes with precise labeling: purity, water content, color index, and chloride content head the spec sheet, ensuring researchers avoid batch-to-batch surprises. In my lab experience, inaccurate water content triggers serious issues for moisture-sensitive reactions; suppliers have started using vacuum drying and argon-packaged containers to combat this. Inventory teams appreciate clear hazard warning icons and QR codes that point to safety data sheets. The compound, classified as a non-flammable liquid, meets most GHS labeling requirements and comes with instructions for secondary containment and proper ventilation.
Preparation Method
Most synthetic routes for N-Ethylimidazolium Trifluoroacetate begin with N-ethylimidazole and trifluoroacetic acid or its derivatives. Ion exchange is key: N-ethylimidazole reacts with trifluoroacetic acid, followed by purification and removal of residual water and base via rotary evaporation or vacuum drying. In process chemistry, column chromatography gives way to phase separation where cost reduction drives scale-up. Crystallization sometimes appears during solvent exchanges, but heats up readily to form a stable liquid. Labs focusing on green chemistry prefer using anhydrous conditions and non-chlorinated solvents. Each step from reactant weighing to final bottling influences chemical purity, with simple process tweaks having outsized impacts on final product performance.
Chemical Reactions & Modifications
N-Ethylimidazolium Trifluoroacetate enables more than solvent-based applications. The ethyl group on imidazole allows for N-alkylation, setting the stage for further cation modification—helpful if specialized solubility or steric properties are sought. The trifluoroacetate portion brings strong electron-withdrawing ability, increasing reactivity with nucleophilic species. Researchers have used it as a medium for palladium-catalyzed cross-coupling reactions, giving consistently higher yields compared to traditional solvents. Its ionic nature can sometimes switch reaction selectivity, influencing ratios of products in substitution and elimination pathways. As green synthesis gains momentum, this compound proves a critical player—both as a reaction medium and as a modifiable platform for designing new ionic liquids with specialized reactivities.
Synonyms & Product Names
Suppliers and journal articles use multiple labels, such as 1-Ethyl-3-imidazolium trifluoroacetate and [C2im][TFA]. Some catalog listings stick with abbreviations like EMIM-TFA. Researchers swapping samples often scribble synonyms on vials, and mistakes happen if folks miss the distinction between imidazolium analogues with different alkyl chain lengths or anions. This highlights the importance of verifying chemical structure before ordering or using in sensitive experiments.
Safety & Operational Standards
Safety teams emphasize protective eyewear and gloves, not because N-Ethylimidazolium Trifluoroacetate means instant danger, but to avoid cumulative irritation or inhalation exposure. In my experience, accidental spills clean up easily with soap and water, but the slick surface can cause slips. Local exhaust ventilation remains a must, as heating can volatilize trace acids present in impure batches. Labs following ISO 9001 standards include real-time monitors to track possible air contamination, and even small-scale users keep MSDS printouts nearby for quick reference. Waste disposal relies on collection domains suited to fluorinated organic liquids; general sink disposal stays off-limits. With proper PPE and fume extraction, risks stay manageable and work proceeds without incident.
Application Area
Industrial chemists and academic researchers put N-Ethylimidazolium Trifluoroacetate to work across a huge spectrum. Its ability to dissolve cellulose supports greener processes for biomass conversion. In catalysis, the compound boosts product yields by stabilizing key intermediates, a feature that single-solvent systems like DMF can't match. Its use as a recyclable extraction solvent streamlines workflows in natural product purification and analytical chemistry. Electrochemists value its conductivity in novel battery and supercapacitor designs, where it supports ionic movement with minimal volatility. In my time collaborating with material scientists, I watched sample preparation headaches melt away as this liquid tackled solubility and phase-separation issues in multi-component composites.
Research & Development
R&D teams at chemical companies race to tune both the cation and the anion in search of new solvents with even lower toxicity and higher thermal windows. Some projects focus on embedding functional groups to create task-specific ionic liquids, aiming to enhance enzyme compatibility or improve metal recovery. In literature, studies reveal strategies to integrate N-Ethylimidazolium Trifluoroacetate within phase-transfer catalysis networks or as carriers for drug delivery systems. Cross-field collaborations multiply as synthetic chemists, computational theorists, and engineers sift through property data to optimize for energy storage, environmental remediation, or pharmaceutical processing. Public funders and private investors keep an eye on eco-impact metrics, directing money to projects that promise real-world gains in process safety and waste minimization.
Toxicity Research
Toxicological assessment of N-Ethylimidazolium Trifluoroacetate has grown as the push toward greener chemistry puts safety under a microscope. Tests so far show minimal acute toxicity toward aquatic organisms compared to classic halogenated solvents. Chronic exposure data still need expansion, especially for scenarios involving large-scale industrial use. In the lab, dermal or mucous membrane irritation occurs at high concentrations but reverses promptly with rinsing. Studies point toward rapid environmental breakdown compared to some stubborn ionic liquids, which reduces concerns over persistent contamination. Regulatory agencies remain cautious, calling for more long-term ecotoxicity data before opening the floodgates for unrestricted deployment.
Future Prospects
Demand for N-Ethylimidazolium Trifluoroacetate will likely surge, driven by policies that phase out toxic, volatile solvents and support renewable processes. Technological breakthroughs could soon unlock cheaper, scalable synthetic methods, cutting costs and supporting broader deployment. I see strong potential in cross-disciplinary initiatives that couple this compound with emerging biorefinery, electronic materials, and catalytic recycling platforms. University-industry partnerships have already sparked pilot projects focusing on closed-loop solvent systems that cut hazardous waste to near zero. Direction from global safety organizations should keep the research pipeline flowing, balancing innovation with clear environmental stewardship. As the chemical landscape reforms, this ionic liquid looks ready to claim a starring role, offering practical, measurable benefits in a world that now prizes sustainability alongside scientific progress.
Digging Into Its Role
N-Ethylimidazolium trifluoroacetate sounds like a mouthful, but it’s quietly playing a big role behind lab doors. For the folks who spend their days looking through beakers and chasing cleaner, greener ways to make chemical reactions happen, this compound stands out. I remember in grad school, everyone scrambled to find solvents that didn’t send us ducking for cover from the fumes. Ionic liquids like this one felt like getting handed a tool that finally solved two problems at once: performance and safety.
Green Chemistry Gets a Boost
Lab safety rules tell a story about how things have changed over the years. Old-school solvents once owned the stage. They work, but the price gets paid through toxic waste and hazardous workspaces. N-Ethylimidazolium trifluoroacetate belongs to the class of ionic liquids, and its chemical properties make it interesting. Instead of evaporating and stinking up a room, it barely makes a peep. Low volatility translates to less air pollution. Many chemists, especially those working on biomass conversion or the breakdown of tough plant matter, have turned to this compound.
What makes it suited for these jobs? Its unusual mix of stability and willingness to dissolve both polar and non-polar substances. Imagine trying to get oil to mix with water—most solvents handle one trick. This ionic liquid can often handle both. In transforming wood chips or straw into something useful, researchers have turned to N-ethylimidazolium trifluoroacetate to help break down lignin and cellulose. It slips between molecules that refuse to talk to each other, bringing them together for new reactions.
Supporting Cleaner Manufacturing
Pharmaceutical labs are notoriously picky about purity, yield, and what ends up in the trash bin. N-ethylimidazolium trifluoroacetate steps up in synthesis steps where tough bonds need breaking or selective reactions are required. I’ve seen chemists swap out more hazardous chemicals for this ionic liquid. Less dangerous byproducts, lower fire risk, and, in many cases, recycling the solvent itself—it’s hard to argue with an approach that helps labs save money and stay out of trouble with regulators.
Some of the most promising studies have used this compound to recycle biomass or handle delicate transformations of organic molecules. Food for thought, especially for anyone alarmed by the amount of waste pouring out of research settings every year. One lab report after another now highlights the shift towards these liquids, marking a real movement beyond talking about “green chemistry” as a buzzword.
Watching for Drawbacks and Pushing Solutions
Costs and long-term impacts always need a close look. N-Ethylimidazolium trifluoroacetate isn’t the world’s cheapest reagent. Scale matters. Research labs might find it within reach, but manufacturers chasing pennies could hesitate. There's also ongoing research into what happens to ionic liquids after their job is done. It’s easy to praise their lower toxicity, but breakdown products require careful tracking. Some countries already push for closed-loop systems to minimize chemical losses and study end-of-life effects.
The next step means recycling the solvent or engineering versions that break down into harmless byproducts. Collaboration between labs, regulatory agencies, and chemical manufacturers looks more important than ever. Seeing green chemistry principles migrate out of academic papers and into factory floors shows that compounds like N-ethylimidazolium trifluoroacetate are more than clever solutions—they’re signs that chemistry’s future lies in daily, mindful choices.
Putting the Pieces Together
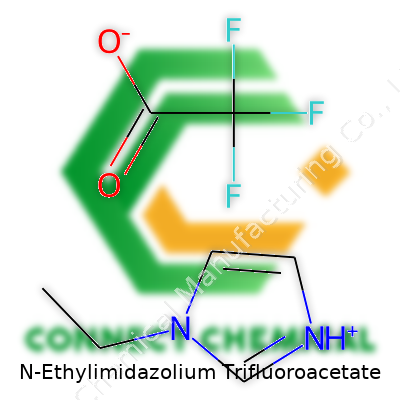
N-Ethylimidazolium trifluoroacetate stands as a modern choice in advanced chemistry labs, often popping up in discussions about ionic liquids and green solvents. This compound breaks into two main parts: the N-ethylimidazolium cation and the trifluoroacetate anion.
Imagine imidazole, a five-member aromatic ring with two nitrogens, found in many biological and synthetic molecules. Add an ethyl group onto a nitrogen at the one-position. Now, this N-ethylimidazolium unit carries a positive charge, making it a cation. Its structure looks like this: a ring of three carbons and two nitrogens, an ethyl chain hanging off one nitrogen, and the whole ring bathed in a bit of positive character.
Trifluoroacetate brings the counterweight, shaped by a central carbon tied to three fluorines and double-bonded to an oxygen—CF3COO-. That negative charge sits on one of the oxygens, heavily influenced by the electronegativity of those tightly-held fluorines. Slap these two units together and they balance out the charge, giving a salt with a unique personality.
Where Chemistry Meets Real Life
Back in college, my organic synthesis group handed off a small bottle labeled N-Ethylimidazolium trifluoroacetate. The task: find a solvent for a tricky cyclization reaction. Oily, almost scentless, it handled water exposure better than most organics I’d used. Working with this compound, I realized the excitement around ionic liquids isn’t just hype. They often show low volatility, low flammability, and break from the terrible trade-offs of traditional solvents—smelly, toxic, and quick to evaporate.
Labs treat this salt as more than a solvent base. The imidazolium backbone, thanks to its stability and ability to easily swap ions, creates new opportunities for catalysis. In recent years, chemists leaned on such ionic liquids to dissolve cellulose, develop new pharmaceuticals, and even study fundamental ion dynamics for next-gen batteries.
Scientists have proven ionic liquids bearing N-alkylimidazolium cations, especially with functionalized anions like trifluoroacetate, encourage cleaner techniques in synthetic chemistry. Reports in Green Chemistry and ChemSusChem journals back this up: reactions performed in these systems produce less waste and sometimes reach higher yields, reducing environmental impacts. Fluorinated anions do raise eyebrows—persistent organic pollutants represent a big problem—but studies reveal trifluoroacetate offers a manageable balance between performance and ecological concern compared to heavier perfluorinated chains.
A Few Rough Edges
No chemical comes without baggage. Trifluoroacetate salts demand respect. Overuse of fluorinated chemicals can poison waterways if not recycled or disposed of carefully. Labs using this compound invest in good waste handling, setting filters and neutralization steps that snatch out fluorinated residues. This isn’t just about rules. Any student who’s worked a fume hood on clean-up day feels the sting if disposal steps get ignored.
Researchers keep eyes open for alternatives. Some are tweaking the imidazolium ring to work with less persistent anions. Others try recycling spent liquids to stretch every last gram. These efforts reflect a growing awareness that chemistry’s future depends on people in the lab making mindful choices—not chasing speed or short-term cost at the planet’s expense.
Understanding the Risks
N-Ethylimidazolium trifluoroacetate isn’t something you stumble across at the local hardware store. Folks working in labs know that handling chemicals like this takes more than a label and wishful thinking. It’s built out of an imidazolium ring with some exotic fluorinated pieces, and that combination means you’re dealing with something that reacts to moisture and heat.
Lessons from Real Life Work Benches
Every scientist has a story about a reagent turning from asset to headache because of sloppy storage. The cue for N-ethylimidazolium trifluoroacetate: any whiff of dampness, and you’re going to wreck a whole batch. Moisture creeps in quick. Watch the humidity — dropping a few milliliters of this material in the wrong place means expensive mistakes, ruined experiments, or even unexpected fumes.
Keep this chemical in an airtight container. Skip the cheap plastic snaps; glass bottles with vacuum grease, Teflon-lined caps, or even crimped vials stand up better over time. Throw in several silica gel packets as backup. Water molecules sneak through unnoticed and then you’re in damage control mode before you even know what hit you.
Control the Conditions
Temperature swings spell trouble for most ionic liquids, and trifluoroacetate salts amplify that risk. I’ve seen folks ignore manufacturer’s advice about cool, even temps — only for their bottles to leak or form crusty residues. The magic number hangs around 2–8°C, which sounds like a regular refrigerator. Don’t just park it next to yesterday’s leftovers. Residential fridges attract moisture every time the door opens.
A laboratory fridge with controlled humidity and a stable shelving system keeps accidents at bay. Label everything. Use clear tags with product name, date, and who opened it last. Trust slips quick when team members can’t figure out what’s inside a mystery jug.
Light also kicks off slow degradation for many chemicals with fluorinated groups. I make a habit of using amber-tinted glassware, or wrap bottles with aluminum foil if options run short. UV breakdown adds up and things go south before you can spot problems.
Prioritizing Safety and Accountability
Handling a reagent safely calls for thinking about clean-up before it hits the benchtop. Spill kits belong close by. Proper PPE — gloves, safety goggles, and lab coats — never gets old, especially with anything carrying trifluoroacetate. People get lazy. The real risk lands on those who think a bit of skin contact or a minor whiff won’t matter. Every chemical safety incident I’ve witnessed involved someone cutting corners "just for a second."
Waste containers labeled for this specific class of chemical keep bigger risks out of ordinary trash. Cross-contamination isn’t just a scare word; you avoid accidental mixing and mystery reactions later on.
Keeping Records and Training Current
Nobody operates alone in a research environment. I’ve seen a dozen close calls nipped in the bud because somebody double-checked incoming shipments, logged their weights, or flagged cloudy contents. SOPs work if everyone uses them — and refresher training every season reminds folks what’s at stake. Treating chemicals with respect, backed by evidence and a lived-in awareness of what can go wrong, does more for safety than any single product data sheet.
Putting Lab Safety First
N-Ethylimidazolium Trifluoroacetate pops up more and more in chemical research. It belongs to the class of ionic liquids, often prized for unique properties—like their ability to dissolve a wide range of compounds and resist evaporation—making them attractive in labs experimenting with greener solvents or electrochemical devices. It sounds cutting-edge, but any time a researcher grabs a new bottle off the shelf, the conversation should turn to safety. There’s a persistent myth in some circles that ionic liquids are almost harmless. The truth feels bumpier.
Toxicity and the Real World
No chemical exists in a vacuum. The safe use of N-Ethylimidazolium Trifluoroacetate depends on understanding its actual risks. Data on its long-term toxicity still looks sparse outside a few academic papers. What we do know comes mainly from the hazards tied to its close relatives: many ionic liquids can disrupt cell membranes, harm aquatic organisms, and cause irritation on skin or eyes. Trifluoroacetate brings another layer. It is a known toxicant at higher concentrations and can stick around in the environment, contributing to concerns around bioaccumulation. People working with the compound typically rely on safety data sheets (SDS) provided by chemical suppliers, but these documents sometimes lag behind the reality in university labs—where students and staff might handle hundreds of milliliters a week.
Why the Details Matter
Many ionic liquids first arrived with promises of low vapor pressure and low toxicity compared to traditional solvents. This reputation led to some relaxed habits. In my own time working in an academic lab, I saw students forgoing gloves around fresh ionic liquids, trusting in their supposed safety. The problem emerges once you realize long-term toxicity studies remain limited and only a handful of groups have pushed for deeper investigation. The fact is, even small molecule exposure, repeated over hundreds of experiments, can add up to a real problem.
N-Ethylimidazolium Trifluoroacetate's possible impact on waterways deserves attention, too. Once washed down the drain, ionic liquids don’t just disappear. Some persist and accumulate in soil and water, threatening the health of aquatic life. Surface water contamination involving fluorinated compounds has led to serious environmental problems in the past, and trifluoroacetate shares properties with more notorious fluorinated pollutants. Europe’s REACH regulations and US EPA guidelines both suggest treating fluorinated compounds with real caution.
Responsible Practices: The Next Step
It’s time for clear communication about the risks of newer lab chemicals—including N-Ethylimidazolium Trifluoroacetate. Professors, researchers, and students benefit from a shared sense of accountability. Gloves, fume hoods, and eye protection shouldn’t be optional when dealing with ionic liquids, whatever the supplier claims. Waste collection should cover anything that could contain traces, not only to protect the user but the local environment. Encouraging supplier transparency gives researchers a chance to demand updated, well-documented hazard assessments.
Chemistry grows safer when the whole community treats every compound—no matter how new or promising—with a hefty dose of respect. Nobody wants to chase yesterday’s safety myths tomorrow, especially when the cost could be measured in health or lost ecosystems.
What Sets N-Ethylimidazolium Trifluoroacetate Apart
N-Ethylimidazolium trifluoroacetate belongs to the family of ionic liquids, which means it stays in a liquid state at room temperature. So, you don't get the familiar fizz of evaporation like with water or ethanol. These ionic liquids have a low vapor pressure, nearly zero, which keeps them from evaporating and filling the room with smells or fumes. That trait helps cut down on workplace exposure and makes experimentation more pleasant, especially if you're used to handling smelly solvents.
What You Feel and See
Pouring this compound, you get a clear, colorless to pale yellow liquid—closer to honey in consistency, not water. That comes down to its viscosity. At room temperature, it flows a bit more slowly than what you might expect if you’ve only worked with simple organic solvents. Viscosity matters because it impacts how easily people mix or process it; as I've learned firsthand, transferring it from one vessel to another calls for a bit more patience.
Despite being a salt, it doesn’t form crystals in your container. That liquid state comes from the combination of its bulky imidazolium ring and the trifluoroacetate anion. Those big ions can’t get close enough to line up and freeze the way table salt does, so the compound remains pourable at lab temperatures.
Odor and Compatibility
There’s almost no scent, a refreshing switch from the sharpness of some organic acids or amines. That low odor makes a difference if you’re working long hours and want to avoid headaches. The absence of vapors aligns with its low volatility. You’re not breathing in much of anything while pouring, pipetting, or cleaning up spills.
And here’s a fact: ionic liquids, especially those based on imidazolium, often dissolve a wide variety of compounds, from salts to organic molecules. That’s true for N-ethylimidazolium trifluoroacetate as well. In my own experience, I found it effective in dissolving cellulose, which almost never happens with standard lab solvents. This broad solubility opens doors for researchers tackling biomass processing and green chemistry projects. Less switching between solvents means faster workflows and less waste.
Thermal Stability and Conductivity
You can heat this compound without worrying about it breaking down the minute you go over room temperature. It can usually hold up until around 200 degrees Celsius before decomposing. Compare that to water, which vaporizes at 100 degrees, and you start to see why ionic liquids like this one help run reactions that need a high heat setting. Its thermal stability plays a direct part in modern synthesis and electrochemistry.
If you hook electrodes into it, electricity flows, but not as easily as in a metal or strong acid solution. The ions move around, carrying charge, though the compound’s viscosity puts a speed limit on that movement. That puts N-ethylimidazolium trifluoroacetate squarely in the running for use as an electrolyte in batteries and supercapacitors, a field that keeps growing as the world searches for greener energy storage solutions.
Safety and Handling
I’ve rarely seen reactivity concerns with this ionic liquid under normal conditions. It resists catching fire or corroding typical lab glassware. But trifluoroacetate ions can react with strong acids or bases, so basic lab safety—goggles, gloves, ventilation—remains the rule. As with many ionic liquids, cleanup takes effort. The sticky texture makes soap and plenty of water essential after spills.
Moving Toward Greener Chemistry
Green chemistry benefits when labs trade out volatile organic solvents for ionic liquids. N-ethylimidazolium trifluoroacetate helps on that front thanks to its low vapor pressure and high chemical stability. But costs run higher, so most labs still pick and choose where this compound fits best. Wider adoption means researchers and producers look for ways to recycle ionic liquids and manufacture them more affordably. That’s often the deciding factor in how fast these substances grow beyond specialty use.