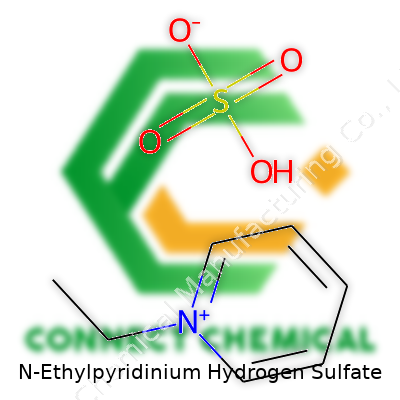
N-Ethylpyridinium Hydrogen Sulfate: An Industry Perspective
Historical Development
N-Ethylpyridinium hydrogen sulfate started attracting attention decades ago. Chemists looked for molecules blending organics with strong acids. Sulfonic acid salts and ionic liquids dominated labs from the late 20th century, but few beat the balance displayed by N-Ethylpyridinium hydrogen sulfate. Research groups noticed its potential for streamlining organic reactions and electrosynthesis before most industries recognized its practical value. I remember work from the early 2000s as case studies. Conversations about “greener” solvents often circled back to pyridinium salts. Universities and industrial teams shared data, showing improved yields and milder conditions when using this compound, especially when compared to harsher mineral acids or legacy reagents.
Product Overview
Manufacturers describe N-Ethylpyridinium hydrogen sulfate as a colorless or pale solid. It smells faintly of pyridine with a slightly acidic note. Popular in both research circles and commercial syntheses, it helps catalyze vital transformations, serves as a component in ionic liquids, and aids electrolyte formulations. Chemists often choose it as a stable, manageable source of sulfate acidity inside organic matrices. Its appeal grew as labs moved away from volatile acids or less controllable reagents. As someone who’s prepped dozens of runs, I appreciate its consistency in behavior batch after batch, especially where predictability means less troubleshooting.
Physical and Chemical Properties
N-Ethylpyridinium hydrogen sulfate crystals appear white and dissolve readily in water, alcohol, and polar aprotic solvents. Heating or storing under open air leads to gradual moisture uptake. Its hygroscopic character marks one challenge—ambient humidity creeps in if you ignore cap discipline on sample bottles, and that changes the weight and behavior during synthesis. Dissolved, the compound offers a mildly acidic solution, with a pH settling lower than neutral but safer to handle than strong mineral acids like sulfuric or hydrochloric. Chemically, the molecule combines electron-rich aromatic properties from pyridine with the robust acid supplied by the hydrogen sulfate anion, forming a reliable acid source for catalysis. The melting range sits close to room temperature, and pure samples hold up under moderate thermal stress, especially when dried and stored away from light and air.
Technical Specifications and Labeling
Distributors deliver N-Ethylpyridinium hydrogen sulfate with purity listed at or above 98%, with trace moisture representing the biggest impurity. Labels usually note identity checks by NMR, IR, and mass spectrometry, and suppliers print warning pictograms for corrosivity and skin irritation. Each drum or smaller jar features a lot number for quality tracking, and certificates of analysis cover melting point, loss on drying, and endpoint titration results for sulfate content. These details help any technician cross-check new lots before using them in regulated syntheses or analytical routines.
Preparation Method
Labs start with pyridine and ethyl bromide, coaxing them together in the right solvent to create N-ethylpyridinium bromide. Next, they treat the salt with silver sulfate in water, exchanging bromide for hydrogen sulfate while removing insoluble silver bromide by filtration. I’ve run this preparation in teaching labs; careful stirring, clean glassware, and glove use make all the difference. Purification by recrystallization or solvent washing sharpens product quality. Drying under vacuum rounds out a sample ready for analytical confirmation.
Chemical Reactions and Modifications
N-Ethylpyridinium hydrogen sulfate handles acylations, esterifications, and transesterifications with enough acidity to drive conversion, yet avoids harsh side-products common with free acids. You’ll find it in select Friedel-Crafts reactions or as a proton source in alkene hydration. Modifications target the alkyl group or the pyridine core, substituting at the nitrogen position to create similar catalysts with altered solubility or reactivity. Swapping out the ethyl chain or introducing electron-withdrawing groups on the ring provides tailored activity for research settings, helping chemists fine-tune processes without turning to less stable or less safe acid reagents.
Synonyms and Product Names
The chemical carries names like N-Ethylpyridinium bisulfate, 1-Ethylpyridin-1-ium hydrogen sulfate, and even 1-Ethylpyridinium sulfate. Each name points to the same balance of ionic organic cation paired with a strong acid anion. Among chemical catalogues and published patents, these synonyms help researchers bridge language gaps in the literature and connect commercial listings. Sometimes, product numbers from suppliers stick for years as shorthand in busy labs.
Safety and Operational Standards
Handling this reagent means gloves and eye protection come out every time. Splashes sting the skin, and inhalation of dust should be avoided—respirators fit for corrosive dusts stay nearby in case of spills. Labs relying on this compound stick to local safety rules: chemical hoods for weighing and transfer, secure dry storage, and emergency shower access for larger spills. Every container leaves the factory with a safety data sheet naming hazards, exposure control methods, and recommended waste handling. Disposal relies on neutralization followed by dilution into industrial wastewater streams, never poured straight into the drain. Keeping records ties into lab accreditation, and annual training refreshes these practices for every technician and student.
Application Area
Organic synthesis stands as the leading use. Process chemists and academic researchers keep it on hand for ester and ether cleavages, polymerizations, and as a proton source in ionic liquid mixtures. Electrochemical cells sometimes call for its inclusion as a conducting salt, where it supports stable voltage and low resistance. In recent years, battery companies probed its role in next-generation electrolytes and ionic liquid solvents, attracted by good thermal properties and moderate environmental impact. More established processes employ the compound in pharmaceutical intermediate production, where its salt form simplifies work-up and reduces corrosion risks inside reactors compared to free acid alternatives.
Research and Development
Chemists in university and startup labs chase novel catalysts with even better selectivity, and N-Ethylpyridinium hydrogen sulfate serves as a reliable benchmark. More projects now mix it with deep eutectic solvents or room-temperature ionic liquids, driving two-phase systems for difficult separations. Comparing output to standard acid catalysts reveals measurable reductions in side reactions. Grants and academic-industrial partnerships often list this compound as a key enabling chemical for environmentally friendlier syntheses. Advances arrive quickly, with new publications mapping out tweaks to its structure for selective activation of aromatic compounds, providing both scientific and industrial incentive to expand its range.
Toxicity Research
Animal models show this compound irritates mucous membranes and causes moderate skin reaction. Chronic exposures at low levels suggest manageable toxicity compared to mineral acids. Environmental assessments point to moderate biodegradability, but with clear warnings against significant aquatic release. Poison control centers treat ingestion with standard sulfate management—hydration and dilution—but rare accidents stress the importance of spill containment and not underestimating respiratory irritation from dust during weighing. Regulatory summary sheets cite Organisation for Economic Cooperation and Development (OECD) toxicity studies, making data accessible for company audits. Workers benefit from regular health surveillance and quick action protocols for spills or accidental contact.
Future Prospects
This compound continues holding the attention of process chemists and materials scientists. Growing interest in “green chemistry” gives a lift to safer, milder catalysts in spots where strong acids once dominated. Renewable and recyclable ionic liquids built from such salts now shape the next chapter of solvent and electrolyte development. As battery research intensifies, companies put more resources into studying these sulfur-containing ionic liquids for their cycle stability and conductivity. Pharmaceutical factories looking to boost process efficiency find these salts appealing for their predictable reaction profiles and manageable safety profiles. Academic papers and patent filings together forecast that N-Ethylpyridinium hydrogen sulfate will keep adapting to new industrial needs as safety, yield, and sustainability become central to chemical manufacturing priorities.
Understanding the Formula: C7H11NO4S
N-Ethylpyridinium hydrogen sulfate goes by the chemical formula C7H11NO4S. Nobody reaches for a bottle of this compound at their local pharmacy, but in real labs, it earns respect for its versatility. It packs a positively charged N-ethylpyridinium ion locked up with the hydrogen sulfate anion. This pairing looks simple on paper, but it’s clever chemistry in action.
Learning Through Experience: Where It Matters
I still remember the first time I handled a pyridinium-based ionic liquid for a synthesis experiment. It was not about just getting the right formula on paper. Every step required careful measurement, attention to safety, and—above all—an understanding of compatibility. Mixing up the ions incorrectly changes the resulting properties, and with hydrogen sulfate in play, one mistake and the whole batch turns unstable. N-Ethylpyridinium’s structure makes it easier to dissolve both organic and inorganic solutes. That unique profile paves the way for practical uses where chemists want an alternative to harsh, volatile solvents.
Facts drive chemistry. Looking at the structure, the pyridine ring connected to an ethyl group gives the molecule versatility. Industries rely on these characteristics to push for greener solvents, better catalysts, and novel extraction agents. Research in ionic liquids highlights the demand for tunable compounds, and N-Ethylpyridinium hydrogen sulfate checks many boxes because of its low volatility and thermal stability.
Potential Hazards Need Close Attention
No one really wants problems spilling into the lab from poor planning. Hydrogen sulfate carries acidity; this isn’t the sort of substance that lets the careless keep all their fingertips. In every chemical stockroom I’ve ever set foot in, proper gloves, ventilation, and chemical-resistant materials make the difference between a smooth day and an emergency call. Safe handling matters as much as knowing the formula itself.
Production runs better with clear safety protocols. The hygroscopic nature of hydrogen sulfate salts means careless storage can lead to product degradation. A simple slip in labeling or a leaky cap, and you face a mess that’s both wasteful and dangerous. Sharing this with younger chemists in training, I often say that a bottle isn’t just what’s inside—it’s every decision made after opening the top.
Better Practices, Smarter Solutions
It’s easy to dismiss chemical formulae as trivia, but N-Ethylpyridinium hydrogen sulfate’s composition points to a bigger conversation about sustainable chemistry. Transitioning away from toxic, flammable organic solvents has brought ionic liquids like this one into focus. Chemistry evolves by looking past legacy processes and pushing for results that don’t demand such a heavy environmental cost. Ionic liquids introduce a middle ground—performance without the hazardous baggage.
Future research depends on creative approaches. Scientists pushing these molecules into new applications—from electrosynthesis work to cleaner separation of metals—need data. My experience matches what literature describes: results hinge not on luck, but on a solid understanding of both hazards and chemistry. Smarter choices and stronger oversight lower risks for everyone in the lab, and the planet outside.
What Sets N-Ethylpyridinium Hydrogen Sulfate Apart
Few folks outside a laboratory have ever come face-to-face with a bottle labeled “N-Ethylpyridinium Hydrogen Sulfate.” Still, this compound plays an understated role in brewing new ideas, making day-to-day products, and hitting quality marks that most people never notice. I remember shadowing a chemist in graduate school who described certain ionic liquids as “the WD-40 of green chemistry,” and this salt fell right into that clutch of chemicals. Its mix of organic and inorganic features brings out special qualities you can't match with typical solvents or catalysts.
Organic Reactions That Need a Gentle Push
N-Ethylpyridinium Hydrogen Sulfate gets a workout in catalysis. Many reactions, especially those creating links between carbon atoms or swapping functional groups, need a gentle push to happen smoothly. This pyridinium salt steps up as an acidic catalyst, speeding up transformations in labs where greener, less hazardous options are welcome. Back when I worked in a university lab, we tested traditional mineral acids and more modern organic catalysts. The old standbys often left a trail of toxic byproducts or tough clean-up jobs. Using compounds like this one helped us shift reactions to safer, simpler conditions, sometimes even letting us skip costly purification steps. Research articles from the Journal of the American Chemical Society have backed up these benefits, noting how organic ionic liquids like N-Ethylpyridinium Hydrogen Sulfate support reactions with higher yields and fewer headaches.
Extracting Value: Solvent Power in Green Chemistry
Aside from catalyzing, this salt pulls its weight as a solvent. It breaks down complex organic molecules, holding them in solution so reactions unfold more readily. At scale, this property supports cleaner processes in pharmaceutical manufacturing, where every efficiency cut means less waste. Many companies now design their chemistry “toolkit” around solvents that are easy on the environment and safe for workers. There’s a powerful argument for investing in alternatives like N-Ethylpyridinium Hydrogen Sulfate, which can cut down on flammable or toxic solvent use. The European Chemicals Agency and regulatory bodies worldwide push for less hazardous chemicals, and this gives salts like this one a real shot in safer, more sustainable practices.
Batteries, Sensors, and Beyond
Not every application centers on making molecules—some focus on moving ions. The conductivity of this compound pops up in energy storage research, including prototype batteries and supercapacitors. Ionic liquids provide the needed stability and charge transport, helping engineers chase better storage life and safety. Research from MIT and other big names in electrochemistry has shown how ionic liquids can boost battery performance without leaking or catching fire, common problems with older battery fluids. N-Ethylpyridinium Hydrogen Sulfate has caught interest thanks to its unique blend of thermal stability and low volatility.
Pushing Innovation While Protecting Health and the Environment
Shifting from old-school solvents and acids to engineered salts like N-Ethylpyridinium Hydrogen Sulfate isn’t just about innovation for its own sake. As someone who has watched a few too many colleagues step away from bench work due to chemical exposure, safer tools make all the difference. Industry trends, journal articles, and my own lab work point toward a future where these compounds help build better medicines, batteries, and specialty materials. At the same time, policy and professional groups—from the Royal Society of Chemistry to the Green Chemistry Institute—encourage more research on health impacts and recyclability. Promoting these solutions means supporting cleaner labs, healthier workers, and less burden on our air and water.
Why Product Care Isn’t Just a Box-Ticking Exercise
People usually see product labels and storage guidelines as just another chore. From years of checking storerooms, handling shipments, and dealing with ruined goods, I’ve learned to treat guidelines as lines of defense for quality and safety. Poor storage and handling often lead to more than a few spoiled items—you’re looking at money wasted, credibility lost, and sometimes safety risks that nobody wants to be responsible for.
Understanding Storage: Keep It Simple, Keep It Safe
Start with the basics: dry products need a cool, dry place, away from direct sun or heat sources. Storing them on pallets keeps items off the ground, helping avoid moisture damage and pest activity. Products sensitive to temperature changes often develop clumping, separation, or lose potency. Foods, chemicals, and even certain electronics can spoil fast with just a few hours in the wrong environment.
In my time working with warehouses, damage usually happened because items sat in humid conditions, near a window, or in corners where airflow suffered. Keeping aisles clear, organizing by date, and rotating stock stops most of those troubles. Temperature monitoring matters too—set up thermometers and do weekly checks, not just when it’s convenient.
Handling With Care: Training Makes the Difference
Rough handling isn’t always obvious. Sometimes it’s the forklift driver who didn’t notice a tipped pallet. Sometimes it’s staff skipping gloves when products need contamination control. Getting hands-on training and updated protocols in place keeps these issues rare. A culture that values asking questions and double-checking saves far more than a high-tech label ever could.
There was a time a supplier tried to cut corners by skipping protective wrapping to save pennies. The losses from damaged goods far outweighed the savings. Simple steps like double-bagging, sealing containers, and keeping items labeled make a real-world difference. Don’t overlook spill kits, handwashing stations, and correct signage—they should never sit in a forgotten corner.
Supporting Facts: What Good Handling Achieves
Food safety data shows improper storage causes over 20% of product recalls. Pharmaceutical storage mistakes often lead to reduced effectiveness, sometimes risking patient health. Electronic goods exposed to humidity develop corrosion and fail testing, increasing customer complaints and returns. Proper practices save resources, reduce reputation damage, and help everyone from top management to the end customer.
Real-World Solutions: Simple Steps to Follow
To keep things straightforward, use color-coded bins or shelves for different risk categories. Create a clear inventory map to cut down on misplacement. Invest in shelves that lift products off damp floors and improve ventilation by rearranging crowded aisles. Assign a storage leader among staff to run checks and receive a small monthly bonus for spotless records—motivation that pays for itself.
Digital inventory systems now let staff set quick alerts for low or high temperatures, making oversight easier. Encourage feedback from all levels—fresh eyes spot the overlooked risks. Most importantly, don’t treat guidelines as mere paperwork: treat them as practical steps that protect your investment and reward your attention to detail.
What Is N-Ethylpyridinium Hydrogen Sulfate?
N-Ethylpyridinium hydrogen sulfate lands in the family of ionic liquids. It doesn’t look like the usual household cleaners or solvents under your sink—this compound gets used in labs and some industrial processes. Chemists like it for its unusual physical properties and its ability to dissolve a wide range of other substances.
The Question of Hazard
Safety stands as a pillar whenever chemicals enter the workroom or the environment. You might think of the most obvious dangers: skin burns, lung irritation, bad smells. N-Ethylpyridinium hydrogen sulfate doesn’t fly entirely under the radar, but public databases and studies haven’t flagged it like they have with notorious troublemakers such as benzene or formaldehyde.
What sets these ionic liquids apart isn’t always visible. Their supposed “green” label attracts folks who want to ditch volatile solvents. Yet, not every ionic liquid stays harmless. Some can linger in the environment. Some hold onto their toxicity, depending on which ions they blend together. This particular compound hasn’t racked up stacks of toxicity reports, but that doesn’t mean it invites casual handling.
Laboratory tests have shown that the ethylpyridinium part of the molecule doesn’t break down fast in nature. Scientists pay special attention to this because lasting chemicals often slip into soil and water, staying there much longer than simple alcohols or acids. Sulfate, by itself, isn’t of deep concern, but the whole compound may bring risks that haven’t shown up in short-term animal tests.
Direct Contact Risks
Anybody who handles N-Ethylpyridinium hydrogen sulfate should use gloves, eye protection, and good ventilation. Direct skin exposure could lead to irritation; getting it in your eyes means a painful, time-consuming rinse. Breathing in mist or powder can crank up coughing or chest discomfort. These effects are straightforward but serious for lab workers and researchers.
Environmental Issues
Fish and insects in local streams may see more of this stuff as labs or industries scale up. The compound lasts longer in the environment than simple salts or alcohols, which builds up risks to creatures lower down the food chain. If cleanup and waste disposal rules get skipped, the residue can end up in groundwater or riverbeds.
My own time working with chemicals shaped my respect for strict storage and disposal. One careless moment, one bottle left open or poured down a drain, lines up to haunt the ecosystem much later. Water tests sometimes turn up residues nobody expected, often far from the original site.
Moving Toward Safer Practice
Some solutions lie in good habits: lock away bottles, wear chemical-resistant gloves, log every use. Facilities can invest in better training and clear labeling. Researchers should push for more tests on long-term toxicity and breakdown in soil and water. Safety improvements don’t need fancy tech—common sense and diligence avoid almost every headline accident.
It helps to loop regulators into the conversation. The patchwork of global regulations means one country’s rules may not match another’s. Eventually, industry and labs need to adapt as new findings trickle out, updating safety sheets and equipment. Anything with unknowns or gaps deserves caution.
Staying Ahead of the Curve
It makes sense to keep a skeptical eye, ask tough questions, push for transparency. The stakes are real for workers and the wider world. Shortcuts almost always end up as someone else’s problem later down the line. With straightforward handling and better research, risks shrink, and the science moves forward.
The Real Meaning Behind “Purity”
Whenever you pick up any kind of product, from table salt to lab reagents, purity isn’t just a number on a spec sheet—it tells you how close you are to getting what you actually paid for. A product boasting 99.9% purity doesn’t leave much room for guesswork. That 0.1% sometimes means something harmless, but it could also be a dealbreaker, especially if you’re dealing with chemical reactions, pharmaceuticals, or anything that ends up inside your body.
Back in my university days, we ran a test with sodium chloride samples. One was fertilizer-grade, another was food-grade. Nutritional uses demand food-grade, but some colleagues shrugged off the difference as marketing. Turns out, the fertilizer-grade sample was tainted with magnesium and calcium salts. It wouldn’t hurt your garden, but nobody wants impurities sneaking into their food or medicine.
Physical Appearance: Not Just for Show
Most people glance at a powder, a liquid, or a tablet and only check if it looks clean and fresh. There’s so much more beneath the surface. Finely milled powder mixes and dissolves well. Off-color samples or clumpy substances? That’s a warning. Contamination or even wrong storage can change color, texture, and sometimes even smell. A batch of vitamin C capsules once arrived with yellowish streaks—normally, pure ascorbic acid stays brilliant white. We later learned the batch was stored near a window and sunlight triggered oxidation.
Physical appearance isn’t always about aesthetics. It can help spot counterfeit or spoiled goods. Pharmaceutical companies employ strict visual inspection for good reason. A single out-of-place tablet might reveal a batch problem—wrong coating, poor mixing, bad drying. Those subtle changes help people on the ground flag issues before a disaster unfolds.
The Stakes in Everyday Life
Purity can make or break outcomes in countless areas. In construction, water with even trace oil affects concrete strength. Bakers fuss about the fineness and color of flour because the bread just won’t rise the same way otherwise. Lab scientists trust reagent-grade chemicals to run their experiments. Slip up, and your report’s useless, your experiment’s ruined, or your business faces lawsuits.
Just two years ago, a supplement company landed in hot water after lab tests flagged traces of heavy metals in protein powders. Fans felt betrayed, and the company’s reputation took a nosedive. Tests later showed that shortcuts during sourcing allowed cheaper, contaminated base powder to slip through. For years, no one questioned the chalky texture or odd taste—until an outside test confirmed suspicions.
How to Raise the Bar
Getting purity right starts with holding suppliers and manufacturers accountable. Certificates of analysis, third-party lab verification, and unannounced audits push everyone to step up. Visual inspection isn’t just for the lab—it pays off in the kitchen, the pharmacy, and the hardware store. Teach teams to trust what they see, smell, and feel. Shared stories about near misses or product recalls drive home the lesson: Don’t take purity or appearance lightly.
Solid regulations already exist for the food and pharma world, but loopholes remain—especially online. Demand transparency. Push for detailed paperwork and test results before you buy, not after. One critical batch can derail a reputation or even claim lives, so cutting corners on purity or skipping physical checks never pays.