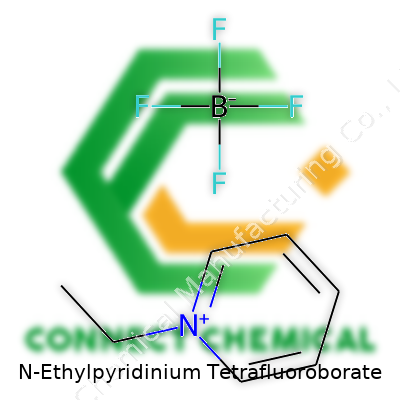
N-Ethylpyridinium Tetrafluoroborate: A Comprehensive Review
Historical Development
Chemists began exploring N-Ethylpyridinium tetrafluoroborate decades ago. Initially, the focus revolved around simple quaternary ammonium and pyridinium salts, especially during the surge in ionic liquid research through the late twentieth century. Lab groups worldwide experimented with pyridinium derivatives for their tunable electrochemical behavior and capacity to stabilize reactive species. After the discovery that exchanging common halide anions for tetrafluoroborate could improve chemical stability and solubility, materials like N-Ethylpyridinium tetrafluoroborate started appearing more often in research papers. Over time, laboratories refined synthetic routes and developed purer batches. Today, both its synthesis and applications show clear hallmarks of this gradual, practical innovation.
Product Overview
N-Ethylpyridinium tetrafluoroborate is a white to off-white crystalline salt, often found in research supply catalogs among other ionic liquids and phase-transfer catalysts. Its most obvious features arise from its charged and stable molecular structure. The ethyl group introduces a modest level of hydrophobicity, while the pyridinium cation core remains highly compatible with polar solvents. The tetrafluoroborate counterion gives the compound outstanding resistance to oxidative breakdown, especially compared to halide alternatives. Researchers know it as a supporting electrolyte in non-aqueous media, and it pops up in benchwork ranging from electrochemical analysis to organic synthesis.
Physical & Chemical Properties
With melting points often reported around 180°C, N-Ethylpyridinium tetrafluoroborate stands up well to heating in most lab settings. The salt dissolves well in polar solvents—acetonitrile and dimethylformamide spring to mind—turning clear without excessive agitation. The boron-fluorine anion resists hydrolysis far better than perchlorates or simple chlorides. If you leave a solution out, it holds up without much decomposition. Its electrical conductivity suits electrochemical experiments, thanks to the mobility of its ions when dissolved. Researchers who worry about competing background reactions or lingering water content trust this salt to stay inert when others would break down or complicate results.
Technical Specifications & Labeling
Bottles often carry specifications such as purity—typically not less than 98%—with label notes on moisture content and storage conditions. Labs pay attention to lot-to-lot consistency. Most suppliers provide melting point, solubility data, analytical spectra, and even supporting NMR references for identification. Good-quality batches look powdery or granular, not lumpy or colored. Labels flag its hygroscopic tendency, so researchers store it with desiccants and avoid long exposure to air. Some safety labels highlight incompatibilities with strong reducing agents.
Preparation Method
Preparation flows from a straightforward quaternization reaction. Pyridine and an ethylating agent—often ethyl bromide—build the pyridinium core with a one-pot process. The final bromide salt dissolves in water, followed by metathesis with sodium tetrafluoroborate. After filtration, drying steps strip away moisture and any byproducts. Chemists who value clean reactions appreciate the minimal side product load, which helps during purification. Scaling up, the straightforward method keeps costs reasonable. Advances in reaction kinetics ensure less time spent waiting for the end point or dealing with incomplete conversions.
Chemical Reactions & Modifications
N-Ethylpyridinium tetrafluoroborate resists many common reaction conditions. It rarely forms new covalent bonds under mild conditions but supports a wide range of redox reactions as an inert scaffold. Some groups use it as an electrochemical mediator, relying on the stable cation and non-coordinating anion. When subjected to strong nucleophiles, its pyridinium ring can undergo functionalization or decomposition. Tetrafluoroborate can, under harsh conditions, release fluoride ions, but the compound holds up to most synthetic chemistries. In specialized organic procedures, scientists sometimes tailor the cation by lengthening the alkyl group to tweak solubility or reactivity.
Synonyms & Product Names
The literature often lists it as 1-ethylpyridinium tetrafluoroborate, sometimes under catalog numbers that hide its identity. Alternative names sometimes shorten the pyridinium core or use ethylpyridinium as shorthand. Some suppliers list it alongside room-temperature ionic liquids, though its melting point stays above ambient. A few research circles substitute the 'N' with '1', making tracking naming conventions important for reproducibility.
Safety & Operational Standards
Handling N-Ethylpyridinium tetrafluoroborate bears similarities to many ionic salts, though sensible precautions apply. Users avoid inhaling dust and ensure gloves touch powder, as both the pyridinium and tetrafluoroborate entities show some toxicity if swallowed or mishandled. Facilities store it away from acids and compatible only with glass or chemical-resistant plastics. In my experience, spills resolve with nothing more than soap and plenty of water, but a full spill kit and fume extraction stand by out of habit. Some protocols advise extra care around open flames and emphasize safe waste disposal to contain fluorinated residues. Safety data sheets from reputable suppliers—Sigma-Aldrich, TCI, and others—outline first aid, proper storage, and recommended personal protective equipment in fine detail.
Application Area
Research and applied science both draw heavily on N-Ethylpyridinium tetrafluoroborate. Electrochemists rely on it as a supporting electrolyte in cyclic voltammetry and other analyses where high cation and anion mobility sharpen measurement quality. Synthetic chemists put it to work as a phase-transfer catalyst or use it to generate reactive intermediates in non-aqueous conditions. Its stability in organic solvents brings it front-and-center in investigations of ionic liquids and electrolyte formulation for advanced batteries or supercapacitors. Emerging uses in photochemistry and specialized catalysis point to a future where this salt plays a key role in tuning reaction outcomes or improving yields.
Research & Development
Ongoing research expands the reach of N-Ethylpyridinium tetrafluoroborate. Scientists evaluate analogs to uncover new reaction partners or build better electrolytic mixtures for energy storage. Recent studies compare the electrochemical windows of various pyridinium salts, looking for the balance between conductivity and inertness. Material science groups test mixtures with other salts to fine-tune ionic conductivity and heat tolerance in solid-state batteries. Collaborative work with computational chemists unpacks solvation shells, helping predict next-generation ionic liquid behavior from first principles rather than slow experimental iteration.
Toxicity Research
Researchers pay real attention to potential hazards, especially given the fluorinated anion in this salt. Toxicity tests on aquatic organisms raise some flags over chronic exposure, driving the need to capture and treat waste streams carefully. Pyridinium-based compounds show moderate toxicity to mammals if ingested or absorbed in large quantities. Most lab work keeps exposures well below problematic levels, but sustained handling under poor ventilation could bring risk. Environmental chemists explore breakdown products to triple-check for persistent, bioaccumulative substances, especially as the use of ionic liquids broadens outside controlled settings. Detailed reviews in toxicology journals encourage manufacturers toward safer synthesis protocols and easier waste mitigation.
Future Prospects
The outlook for N-Ethylpyridinium tetrafluoroborate looks promising as electrochemical devices become more demanding and synthetic chemistry searches for robust, adaptable materials. There is steady interest in transitioning from volatile organic solvents toward safer ionic liquids and designing compounds tailored for high-performance battery electrolytes. Deeper understanding of structure-property relationships guides the push for greener production methods and improved safety. Researchers contribute to a growing toolkit for both applied and theoretical science. N-Ethylpyridinium tetrafluoroborate shows every sign of remaining relevant as new technologies push chemical requirements further, suggesting strong demand for years to come.
Chemists Speak This Language for a Reason
Ask any organic chemist to write out the formula for N-Ethylpyridinium tetrafluoroborate, you’ll likely get a straight answer: C7H10BF4N. Behind that tidy string, though, sits a world of nuance. Chemists tend to respect these little packages of letters and numbers because they make sense of structure, function, and safe handling in one go. Picture a chemistry lab at my old university—brown bottles everywhere, all those strange names, but you only need the formula to pull up research, toxicity, or who you need to call if something spills.
The Nuts and Bolts: Understanding the Formula
The formula C7H10BF4N packs in a fair bit of information for such a small label. There’s the pyridinium ring (six carbons, a nitrogen), an ethyl group hanging off that nitrogen, and a BF4– counterion balancing the charge. Think of it like a story told in shorthand—chemistry notes etched in black marker on aging glassware.
N-Ethylpyridinium tetrafluoroborate doesn’t pop up in daily life unless you live in a world of synthetic chemistry or advanced battery research, but its chemical identity explains its quirks. The tetrafluoroborate anion resists decomposition even in wet air; the organic piece brings solubility in polar solvents. In the field, you chase after these formulas because you need reliable results—the littlest error in the symbol changes the entire outcome. During one project synthesizing ionic liquids, I watched a classmate confuse tetrafluoroborate (BF4–) for hexafluorophosphate (PF6–), only to discover the reaction tanked completely.
What It Means for Safety and Application
Clear chemical formulas become essential when it comes down to safety sheets and experimental protocols. Google “N-Ethylpyridinium tetrafluoroborate” and you'll pull up hazards, disposal guidelines, and handling rules in an instant. The formula signals both possibilities in the lab and dangers if someone ignores the rules. The nitrogen in the ring and the boron-fluorine cluster serve as red flags for anyone proper-trained—don’t inhale dust, keep the hood running, glove up before working.
Research and Responsibility
College chemistry hammered into me the importance of E-E-A-T—not just in search, but in how we treat and trust information. Expertise shows up in correct formulas; authoritativeness comes from peer-reviewed data; trust arrives with accurate labeling. One professor insisted that we check every bottle twice, because a missing atom or swapped ion can lead to wasted time, failed experiments, or worse, a dangerous accident.
N-Ethylpyridinium tetrafluoroborate’s formula might look dry on a page, but it plays a real role in technology, whether that’s making room-temperature ionic liquids or helping design new batteries. For every innovative material out there, the precise chemical formula sits at the root, carving out a path between ambition and safe progress.
A world without careful chemical notation would be chaos in the lab and industry. Lessons from hands-on experience leave a clear takeaway: chemistry happens in the details, and those details sit inside every chemical formula, guiding both innovation and the standards that keep us working safely.
A Key Supporting Role in Organic Synthesis
Every chemist knows the challenge of finding the right ingredient to pull off an efficient reaction. N-Ethylpyridinium tetrafluoroborate shows up as a favorite in countless research labs, mainly because of its solid performance as an electrolyte and alkylating agent. When I worked in a university organic chemistry lab, we often sought out reagents that stayed stable in harsh conditions and didn’t complicate reaction cleanup. N-Ethylpyridinium tetrafluoroborate struck the right balance—reactive enough to push reactions but not so volatile that it brought in strange byproducts.
Advanced Electrochemistry
A growing group of battery researchers and industrial electrochemists reaches for this compound as a supporting electrolyte. Its popularity comes from its chemical stability and its ability to dissolve well in a wide range of solvents. Once, we needed to set up a tricky cyclic voltammetry experiment on a new catalyst, and almost every published protocol pointed to N-Ethylpyridinium tetrafluoroborate for a smooth and consistent response. In practice, using it led to reproducible results and very little noise, which meant our data on that expensive catalyst remained readable and trustworthy. For anyone in redox chemistry or advanced electronics research, having a reliable electrolyte defines the experiment’s quality.
Alkylation Reactions: Clean and Reliable
In organic synthesis classrooms and industry, alkylation reactions deserve special mention. Adding an ethyl group to a molecule seems simple but can turn into a mess if the reagent decomposes too quickly or forms impurities. N-Ethylpyridinium tetrafluoroborate quietly improves these transformations, giving chemists more control. Studies point to its usefulness in making certain intermediates for pharmaceuticals and agricultural chemicals. By choosing this salt, labs reduce trouble with purification and get better yields—something every lab manager looks for to save money and time.
Smart Design of Ionic Liquids
Ionic liquids matter in both academic papers and green technology labs. Their low volatility and custom properties promise less toxic, more sustainable industrial processes. N-Ethylpyridinium tetrafluoroborate helps craft ionic liquids with unique properties—like improved conductivity or selective solubility—for said tasks. Researchers have used it to design systems for CO2 capture and for extracting valuable metals from waste, tackling tough environmental challenges. Its physical features—high melting point, unusual solubility—open doors to new fields, not as a flashy headline ingredient but as a cornerstone for innovation.
Challenges and Possible Solutions
Anyone working with these chemicals knows they don’t come risk-free. Fluorinated salts, including tetrafluoroborates, can give rise to waste that needs careful handling. The move to greener chemistry means companies need strong waste management plans or ways to recycle used reagents. Exploring alternatives with less hazardous byproducts could cut down on the environmental cost without sacrificing the strengths that make N-Ethylpyridinium tetrafluoroborate useful. In academia, safety training and up-to-date protocols keep harm at a minimum, but consistent funding for such measures supports safer labs everywhere. As interest in high-performance batteries and cleaner manufacturing rises, demand for safe and responsible use of these salts will likely grow even more.
Practical Handling and Safety Priorities
N-Ethylpyridinium tetrafluoroborate comes up in chemistry labs usually due to its role in specialized organic synthesis and electrochemistry. Handling any powdered salt with fluoride connections sets off alarms for ill-prepared personalities. Let’s start with the basics: labs deal with enough hazards even before someone mishandles a chemical like this one. Good practice means keeping containers tight, protected from moisture and open air. Humidity fiddles with stability, and nobody wants a salt clump or unexpected reaction during experiments.
Labels might make this one sound basic, but it isn’t table salt. Swallowing, skin contact, or breathing the dust can set off serious irritation. A decent pair of gloves and some eye protection always beat regret. Fume hoods aren’t overkill—on a rough day, they keep accidental powders away from lungs or desk sandwiches. Respecting chemical boundaries means fewer disruptions in the safety routine, and it keeps lab mates happier.
Temperature, Light, and Shelf Duty
N-Ethylpyridinium tetrafluoroborate stays most stable at room temperature, away from strong sunlight or heater vents. Cool and dry wins the day. If humidity sneaks into the picture, those tetrafluoroborate ions may decompose and form hydrofluoric acid, which nobody wants in a breathing space. It’s worth remembering that HF, even in small amounts, can burn skin and bones deep—making it clear why casual attitudes cause long-term consequences.
Glass or compatible plastic bottles work well for long-term storage. Some folks try to save money using whatever container they find, but that risks a reaction with the packaging itself. Costs pale in comparison to the mess a leaky bottle leaves behind or chemical burns from surprise reactions at the bottom of an old jar. Chemicals that sit quietly on a shelf for months can surprise the unwary if ignored or left in sunlight, so a well-sealed, shaded spot serves best.
Emergency and Disposal Sense
Accidents still find their way into everyday routines. Spills on benches or the floor get cleaned up with gloves, disposable towels, and a careful eye. Folks should always check that chemical spill kits are stocked for fluoride-containing powders. If the chemical winds up on skin, water rinses off most of it. But waiting on emergency help after direct contact with hydrofluoric acid by-products makes everything worse—fast first-aid action becomes essential.
Disposal brings another level of respect. Pouring anything containing fluoride ions down the drain rarely ends well, both for building plumbing and the wider city environment. Waste teams with chemical disposal training keep everyone out of trouble, and fielding questions to a licensed waste handler does not stain anyone’s reputation.
Why All This Care Matters
Lab chemistry has always balanced curiosity with caution. The extra effort to store N-Ethylpyridinium tetrafluoroborate in a dry, well-marked, cool place makes daily routine less stressful. Thinking through PPE and responsible waste disposal avoids late-night emergencies that nobody enjoys dealing with. People learn their lessons either by following the facts or by cleaning up after someone else’s mistake. One safe day often means more time for discovery, and less time inventing reasons for safety officers to drop by.
What’s in a Chemical Name
Every lab-minded person, at some point, comes across chemicals with long intimidating names. N-Ethylpyridinium Tetrafluoroborate doesn’t show up on most kitchen shelves, but folks in chemistry departments and high-tech industries recognize it as a salt used in specialty syntheses and electrochemical experiments.
What We Know About This Chemical
Some researchers treat this compound as another reagent for research. The molecular structure pairs an organic cation, N-ethylpyridinium, with the BF4- anion. That’s a mouthful, but it simply means: one part comes from a pyridinium ring with an ethyl group stuck on, and the other is a boron-fluorine cluster. The combination offers chemical stability, which is why it sees use in electrochemical cells, ionic liquids, and sometimes in organic synthesis.
Hazards must always be discussed with context. Unlike classic “danger signs” like mercury or benzene, N-ethylpyridinium tetrafluoroborate doesn’t jump out in common toxicology lists. The chemical literature, including common safety databases like PubChem or Sigma Aldrich, points to caution: suppliers label it both toxic and irritating. Inhalation, ingestion, or skin absorption could be harmful. The boron-fluorine pairing, especially, poses some risks if it’s involved in high-heat or moisture-prone situations, potentially releasing toxic fluorine compounds.
Why It Deserves Respect in Handling
Comfort in the lab sometimes leads to underestimating what’s in the bottle. I’ve seen that overconfidence cost students time and safety. No matter how mild a compound appears from a handful of case studies or sparse animal data, chemicals like this deserve respect. Fluorinated salts, as a class, can create hazardous fumes. Burning or reckless mixing sometimes produces hydrogen fluoride, a chemical with a nasty reputation for causing burns that go straight through skin into deeper tissues. A researcher pouring this chemical outside the fume hood might not see fumes, but could end up feeling irritation or worse.
Boron isn’t as scary as mercury or cadmium, but even “low-toxicity” elements can be trouble in the right context. The biggest concern with this compound comes from chronic or high-level exposure. It’s easy to let down your guard when published research papers list it without hazard warnings, but that doesn’t erase potential harm with repeated exposure.
Backing Up Precautions with Real Practice
Safety data sheets matter, but lived experience gives the best lessons. Anyone handling unknown or specialty compounds should rely on good ventilation—preferably a fume hood. Nitrile gloves, safety goggles, and lab coats never go out of style. Even after a “safe” day’s work, handwashing can prevent residue getting into a sandwich at lunch or onto a phone screen. Once, during a project with less exotic quaternary ammonium salts, a forgotten glove led to strange skin irritation; it turned out one labmate hadn’t changed gloves after touching an unlabeled flask.
Waste disposal also raises eyebrows. Tossing leftover residues in the sink risks contaminating downstream water. Local regulations help determine the right approach, but most labs send this type of chemical to hazardous waste, away from public water or landfill sites.
Managing the Risks Is Possible
Some worry that every new reagent spells out disaster for green chemistry, but with proper gear and habits, the risks of N-ethylpyridinium tetrafluoroborate become manageable. The facts say: don’t eat, breathe, or spray it around; keep it sealed and ventilated; and treat all unfamiliar chemicals like they might bite. Conversations with safety officers, and reading SDS sheets in full, help everyone stay honest about the hazards. This approach keeps research moving and, most importantly, keeps people healthy.
Understanding What You’re Getting
Working in a lab, I’ve seen plenty of cases where the difference between a half-decent result and a promising breakthrough comes down to the quality of the chemicals on the bench. N-Ethylpyridinium Tetrafluoroborate isn’t just a mouthful—this compound finds its way into organocatalysis projects, electrochemical research, and other applications where a stray impurity can throw off the whole show.
Most suppliers offer it at a purity of 98% or higher. Reaching that level means strict controls during synthesis and meticulous purification. In my experience, the extra cost for a batch that guarantees over 99% purity feels justified, especially for sensitive reactions or analyses where a little contamination can mess up months of work. Always ask for a certificate of analysis and read the fine print. Some companies really do skimp on documentation or use generic test methods that leave you guessing about what’s in your reagent bottle.
The Numbers Behind the Bottle
The most common packaging sizes cover a range suitable for research and development as well as process-scale work. You’ll find 5-gram, 10-gram, 25-gram, 100-gram, and even up to 500-gram bottles from various vendors. A 5-gram bottle goes a long way in a small analytical or synthetic run; for scale-up, the 100-gram or larger packaging saves money and cuts down on constant reordering.
One frustrating point: Sometimes suppliers only sell large sizes, or require a special order for anything under 25 grams. This isn’t just annoying—it hits university and startup budgets hard when you only need a little. In the past, I’ve reached out to suppliers directly and negotiated smaller quantities. They’ll sometimes repackage from bulk or suggest co-shipping with other customers, especially if you’re in a research consortium or university network.
For storage, this salt is generally sold in amber glass bottles or sealed HDPE containers, often under inert gas. Moisture and air can cause hydrolysis or slow degradation, so don’t trust a supplier who cuts corners with cheap plastic jars or loose caps. Cross-check the packaging recommendations; reputable producers care about shelf-life for a reason.
Why Purity and Size Choices Affect Research Progress
I’ve seen what happens when colleagues cut corners on reagents. Even a small impurity can tank an electrochemical measurement or spoil a catalysis reaction. Literature reviews on N-Ethylpyridinium Tetrafluoroborate highlight the impact of low-level byproducts on reproducibility. In academic research, clean results matter for publishing and peer review. In industry, process reliability gets measured in dollars lost or saved.
Waste is another issue. Oversized packaging leads to expired stock sitting on shelves, while too-small bottles slow down work and ramp up costs. I’ve used collaborative purchasing groups—especially in university or biotech incubator settings—to match needs to supply. In my last lab, group orders let us access high-purity stock, split costs, and cut down on unnecessary leftovers.
Better Options for Scientists
Open communication with suppliers brings better outcomes. I recommend asking for detailed batch analysis, including data like NMR and ICP-MS reports if available. For small-scale users, pushing suppliers to provide custom-sized packaging pays off in the long run. Some companies have begun offering subscription models or quarterly shipments for frequently used chemicals, which helps keep the workflow steady.
In my career, paying attention to little details like purity and packaging size turned out to matter as much as technique or fancy equipment. Solid choices start with knowing what you’re buying and demanding what you need. That’s how good science keeps moving forward.