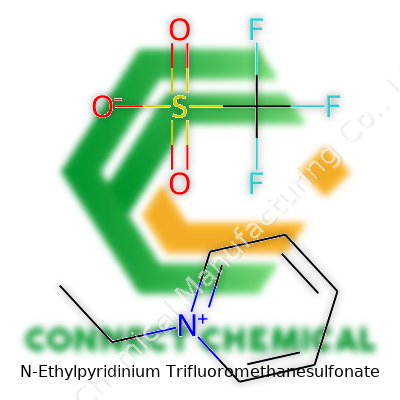
N-Ethylpyridinium Trifluoromethanesulfonate: Beyond the Lab Bench
A Journey Through Its Origins
Chemistry builds on the steps of those who came before, and N-Ethylpyridinium Trifluoromethanesulfonate draws from decades of pyridinium research. Back in the early 20th century, scientists started to see value in pyridinium salts, tracing their properties through both academic and industrial curiosity. As synthetic techniques advanced, the addition of the trifluoromethanesulfonate group unlocked a new tool for chemists aiming for stronger acids and ionic materials. This compound did not arrive overnight; it represents patience, repeated failures, and the drive to make molecules that push the edge of what’s possible in synthesis and catalysis. Its story folds into the broader arc of ionic liquid development, especially as green chemistry grew from niche interest to central doctrine.
What the Product Offers
N-Ethylpyridinium Trifluoromethanesulfonate comes as a solid, sometimes crystalline or powder, and offers high solubility in polar solvents. I’ve seen it packed in moisture-proof bottles, which matters because triflate salts tend to pull water from the air and their quality drops if storage isn't tight. The compound pairs a positively charged pyridinium ring, tweaked with an ethyl side chain, with a triflate anion—known for its electron-withdrawing punch and chemical stability. This setup lets chemists exploit both the cation and anion’s traits, tackling problems around conductivity, ionic liquids, and forcing tough chemical changes in stubborn substrates.
Physical and Chemical Hallmarks
In terms of melting point, you’ll find numbers hovering around 160°C, but the exact figure dances with traces of water or impurities. The triflate anion keeps it thermally robust—no wild decomposition until higher temperatures. The compound dissolves with ease in water and most organic solvents, which sets it apart in the realm of ionic salts. That stability tracks with the triflate’s fame for resisting nucleophilic attack and holding up in strong acids or bases. In the lab, I’ve watched it shrug off conditions that send other salts into stinky breakdown products.
Technical Specifications and Labeling
Every reputable supplier lists batch-specific technical details, hitting benchmarks for purity, moisture content, residual solvents, and trace metals. Typical analyses show purity above 98% and minimal coloration. Each bottle sports clear hazard labels—signal words like “Warning” and pictograms showing possible irritant effects, so everyone knows what gloves and eyewear to grab. The labeling should lay out not only chemical formula, but also storage temperature (usually cool, dry shelves), and expiration or retest dates. Skimping on these details means risking ruined experiments or dangerous exposures.
Preparation: How Lab Benches Bring This Salt to Life
Synthesis starts with N-ethylpyridine, a derivative of the basic pyridine scaffold. Using an alkylation route, chemists react the nitrogen with an ethylating agent—usually ethyl halide. After it picks up its extra carbon chain, the resulting pyridinium halide is tossed into the ring with silver triflate. That silver salt ensures only triflate anions come out in the end, leaving a precipitate of silver halide as a byproduct. Each wash and washback along the route strips away excess reagents and side products, followed by careful vacuum drying. The final salt smells subtly aromatic and flows with the gritty looseness you find in many organic ionic solids.
Chemical Reactions: What It Can and Cannot Handle
N-Ethylpyridinium Trifluoromethanesulfonate stands out in several organic reactions, especially as an ionic liquid component, a phase-transfer catalyst, or a support for strong acid reactions. Its combination of charge and stability helps shuttle reactants between aqueous and organic layers—no chunky emulsions, just fast, clean mediation. You rarely see it acting as a nucleophile since both the cation and the triflate refuse easy electron donations. Researchers have leaned on the compound for activating carbonyl groups, and promoting alkylations and Friedel-Crafts acylations. Chemical tinkering on the pyridinium ring offers a playground for new functionalities, by switching side chains, attaching fluorinated groups, or even linking up with other ionic liquids. Its chemistry keeps stretching outward as labs explore greener, more selective synthesis tools.
Other Names and Market-Friendly Titles
Rounding up synonyms helps avoid confusion—one of the most common alternative names remains 1-Ethylpyridinium Trifluoromethanesulfonate. Some catalogs simplify it to ethylpyridinium triflate or N-EtPyOTf. Over the years, manufacturers have streamlined trade names for clarity, skipping confusing abbreviations so nobody mistakes it for related pyridinium salts equipped with different anions.
Staying Safe and Following the Right Standards
Working with any pyridinium-based ionic salt brings safety concerns, especially with skin and eye exposure. The triflate doesn’t ramp up acute toxicity but the cations can act as mild irritants or pose long-term issues with repeated contact. Labs need routine PPE—gloves, goggles, dust masks where handling powders spills risk. I’ve seen spills handled with absorbent paper and a trip to the hazardous waste bin, every jar clearly labeled with GHS-compliant stickers. Good ventilation helps prevent inhalation. No matter how much experience someone has, overlooking safety with pyridinium salts risks both accidents and ruined work.
Where It Shows Its Strength
N-Ethylpyridinium Trifluoromethanesulfonate earns respect in organic synthesis, mostly where selective ion pairing or tough catalytic conditions matter. Ionic liquid fans know it for non-volatile media design—something greener than historic chlorinated solvents. In research, it plays a role in electrochemistry, low-melting molten salt systems, and battery studies. Pharmaceutical labs find it handy in making highly polar transition states or in isolating certain intermediates. Whenever better phase separation or conductivity is required, this salt routinely makes the shortlist.
On the Frontiers: Research and Ongoing Development
Recent grants in physical organic chemistry and material science often mention N-Ethylpyridinium Trifluoromethanesulfonate for custom ionic liquids—mixing and matching cations and anions with hopes of outperforming legacy solvents and supporting next-gen catalysts. Research groups have zeroed in on designing task-specific ionic liquids for separating rare metals, desalination, and green organic transformations. Teams in academia and industry track purity, stability, and functional switching to squeeze more life from the basic pyridinium-triflate structure. I’ve watched postdocs present posters showing improvements in energy storage, cleaner peptide synthesis, and cutting solvent waste with these materials.
Toxicity: Learning Where to Draw the Line
Any new chemical in the lab needs a toxicity profile, especially with ionic salts known for bioactivity. Pyridinium cations— depending on substitutions — can slip into cell membranes. Studies so far have not flagged N-Ethylpyridinium Trifluoromethanesulfonate for high acute toxicity, though long-term exposure or improper disposal can add up. Environmental fate studies hint at persistence, since triflates resist breakdown in water and soil. That means chemists favor closed systems and routine hazardous waste protocols over care-free sink disposal. Regulatory agencies expect clear documentation on safe handling, emergency spills, and first-aid procedures.
Where the Road Leads: Future Uses and Questions
Prospects for N-Ethylpyridinium Trifluoromethanesulfonate stretch far past traditional organic labs. The ionic liquids boom sends ripples through battery technology, extraction science, and medicinal chemistry. Fine-tuning its molecular edges for greener syntheses and targeted catalysis sits high on many to-do lists. The drive for more biodegradable ions and faster catalytic tweaks points to years of synthetic and application tinkering ahead. As climate concerns loom larger, green chemistry will push the boundaries on how and where these salts offer both efficiency and sustainability. On a personal level, watching a once-niche material become a staple across so many research fronts leaves no doubt—sometimes the biggest advances start with small, purposeful steps.
Understanding the Pieces
There’s a moment, staring at a chemical structure, when all the separate atoms and bonds catch your attention. N-Ethylpyridinium trifluoromethanesulfonate has two distinct parts. Take the pyridinium ring, swap a hydrogen for an ethyl group at the nitrogen. This positively charged ring draws researchers because it almost feels like a small platform for all sorts of chemical tricks. The ethyl group, which connects right to the nitrogen, flips the character of the usual pyridine ring. The positive charge creates all kinds of opportunities for pairing with negatively charged partners.
The counterion here—trifluoromethanesulfonate, also known as triflate—brings stability. The triflate anion has a carbon attached to three fluorines and a sulfonate group. With formula CF3SO3−, triflate’s broad electron cloud makes it not only stable, but quite good at dissolving in organic solvents. The pairing of these two entities isn’t random. It’s a match that lets scientists explore broader chemical reactions, especially in the world of ionic liquids.
The Structure in the Real World
The N-ethylpyridinium part follows the classic aromatic hexagonal structure of pyridine, all carbons and a nitrogen in a ring. Add the ethyl group to that ring’s nitrogen, and the whole thing gains a positive charge. On paper, it looks simple: the ring, the tail, the positive sign. In practice, this structure directs how the molecule interacts with others and how it dissolves in different solvents.
The triflate anion brings its own quirks to the mix. Its backbone—a carbon surrounded by three tightly bound fluorines and a sulfonate group—pushes electron density away from itself. That means any positive charge nearby, like the one from the N-ethylpyridinium, feels right at home.
Why This Structure Matters
Molecules like N-ethylpyridinium trifluoromethanesulfonate pop up in a lot of labs because their design brings advantages in fields like electrochemistry and organic synthesis. The ability to tweak the properties of a solution just by swapping a group at the nitrogen is a real boon. That ethyl group isn’t there just for show; it changes how easily this molecule can pass into or out of non-polar environments, which can mean faster or more selective reactions.
Triflate’s strength comes from its non-coordinating nature. It usually doesn’t jump into reactions itself, so researchers can use the triflate anion as a passive star—helping with solubility, maintaining ion pairing, but not interfering.
Issues and What Can Be Done
One issue that keeps surfacing relates to environmental persistence. Triflate compounds can be tough to break down, because those fluorines just don’t want to let go. In a world facing PFAS pollution, adding more fluorinated chemicals into systems deserves real scrutiny. Laboratories that use these compounds need clear protocols for waste management, and industries should look at greener alternatives when possible. It helped that chemists continue searching for anions that perform as well as triflate but break down more easily outside of the lab.
Balancing performance, practicality, and sustainability takes cooperation across researchers, suppliers, and regulators. The structure may sit on a piece of paper, but its implications ripple out. A closer look at N-ethylpyridinium trifluoromethanesulfonate’s design shows how structure, function, and real-world impact all travel together in modern chemistry.
N-Ethylpyridinium Trifluoromethanesulfonate: A Hands-On Compound
N-Ethylpyridinium Trifluoromethanesulfonate, sometimes called NEPyTf, finds its way into chemistry labs because it does more than sit quietly on a shelf. This ionic compound, first synthesized for its stable pyridinium core, shows versatility across research and industrial settings. If you’ve ever followed developments in organic synthesis, the push to find better, greener, and more productive catalytic systems can't go unnoticed—NEPyTf steps into this gap.
Driving Organic Synthesis
Organic chemists prize this molecule for its role as a catalyst and ionic liquid. The unique pairing of the pyridinium ion and the trifluoromethanesulfonate keeps NEPyTf stable even under tough reaction conditions. In my own work during graduate school, searching for a better alternative to old-school solvents led to NEPyTf. Its strong ionic character helps dissolve both organic and inorganic materials, moving challenging reactions along without bringing extra hazards that often show up with volatile organic solvents.
Larger pharmaceutical companies keep an eye on reaction outcomes and side products, both from a safety and a regulatory perspective. Using NEPyTf shrinks the risk of hazardous byproducts, especially in acid-catalyzed processes. It has shown success catalyzing esterification and alkylation, two classic reactions that build up bigger, more complicated organic molecules. Cleaner and safer pathways here translate into smoother investigations and easier regulatory approval later down the line.
Roles in Electrochemistry and Energy Research
Stepping into the world of batteries and supercapacitors, I’ve seen researchers turn toward new ionic liquids for supporting electrolytes. NEPyTf, thanks to its strong ionic conductivity, helps shuttle charges in devices aiming for high efficiency and safety. Its thermal stability means batteries can work at higher or lower temperatures without running into breakdown problems. This particular trait keeps popping up in recent academic papers focusing on energy storage—battery reliability still dominates research funding and product design choices.
As new devices emerge, people look hard at how ions move through liquid electrolytes; here, trace amounts of moisture or poorly chosen additives can wreck a project. NEPyTf resists hydrolysis, which means it won’t quickly fall apart in the presence of water. This allows scientists to stretch into new territory without starting from scratch every time moisture sneaks in. That’s a lesson learned the hard way in many electrochemistry experiments—control the variables, or lose hours of work.
Fine-Tuning Analytical Chemistry
In analytical labs, careful separation and detection matter. NEPyTf boosts selectivity and sensitivity in spectroscopic assays—think nuclear magnetic resonance or infrared readings. It’s not the starring role, but as a supporting cast member, NEPyTf strengthens weak signals and makes hard-to-spot differences stand out. Analytical chemists need that edge when teasing apart similar molecules in complex mixtures. This ability to make signals pop out can help in drug development, food safety, and forensic work, where the difference between a false negative and a true result changes lives.
Where To Go From Here
Sustainability always stands as both a challenge and a motivation. NEPyTf still commands a higher cost than basic salts, slowing wide adoption in lower-budget settings. But as companies scale up green chemistry and energy storage research, sharing best practices around this compound will help drive down price and increase adoption. Collaboration between universities and manufacturers fuels most breakthroughs. By directly supporting open research, wider training in lab safety, and circular recycling programs, NEPyTf can become part of tomorrow’s standard toolkit, not just a specialty tool for a lucky few.
Putting Safety Upfront
Chemical storage isn’t just about the label; it’s often about long-term health, stability, and smooth research or industrial workflow. N-Ethylpyridinium trifluoromethanesulfonate packs a punch in synthesis labs and specialty applications, but even experienced chemists don’t always pause long enough to think about its storage in real-world terms. Having handled sensitive chemicals in academic and industrial settings, I understand how small lapses can override best intentions. A dry, cool storage area — free of sunlight and humidity swings — stands at the heart of good chemical health.
Guided by Stability
N-Ethylpyridinium trifluoromethanesulfonate brings strong ionic qualities and is generally stable, but nobody beats the environment. Leaving it in a spot where moisture sneaks in, or temperatures shift up and down, introduces problems over time. A desiccator with fresh desiccant becomes a reliable friend here. Storing this compound in tightly-sealed, labeled containers goes beyond habit; it’s the method seasoned chemists adopt to avoid ruined samples or unwanted reactions. My own time in shared university storerooms taught me the value of double-checking that lids were on tight and labels had dates. These small acts stop headaches weeks or months later.
Keeping Access Simple, Not Risky
No one enjoys wading through clutter or confusion just to grab a few grams for a project. Dedicated shelves in a chemical cabinet work better than tossing jars together on a bench. N-Ethylpyridinium trifluoromethanesulfonate doesn’t have some of the violent incompatibilities seen with acids or strong oxidizers, but it still makes sense to separate it from reactives and water-sensitive chemicals. Cross-contamination hits hardest in high-purity applications where even minor mix-ups waste money and time. In one project, a single mislabeled bottle blended into others could have cost us weeks. Organization isn’t just about neatness—it actively prevents lost progress.
Labeling Keeps Everyone Honest
Every researcher has stories about mystery jars from years past. Marking storage containers with contents, date received, and hazard codes answers questions right away. Older material often gets rechecked or discarded, which saves embarrassment or risk. Training new team members to maintain this labeling discipline pays off quickly, as even the most experienced can get mixed up on a busy day.
Looking Forward: Balancing Cost and Care
It’s tempting to cut corners on environmental controls because air conditioning and desiccators require up-front costs. Yet the savings disappear after a few ruined batches or contamination scares. Institutions and labs should look at the numbers: protecting a few hundred dollars’ worth of specialty chemicals shields projects worth thousands. Smart inventory practices—like regular audits and cycling through stock—extend resources further. From my own experience, tracking expiration dates and regular checks prevent emergencies. Mistakes don’t only waste money; they put people and reputation on the line.
Responsibility: Personal and Collective
Chemical storage culture doesn’t thrive through signs on walls alone. Teams improve results when everyone values careful storage, honest labeling, and prompt cleanup. Sharing personal experiences, good or bad, helps new team members learn without repeating mistakes. At the end of the day, safe, careful storage of N-Ethylpyridinium trifluoromethanesulfonate preserves chemical integrity and builds trust in the lab or workplace. That matters just as much as technical precision in the setting up of any experiment.
Understanding the Risks
N-Ethylpyridinium Trifluoromethanesulfonate turns heads in many chemical labs because of its use in catalysis and organic synthesis. It comes with its own set of risks, like most salts in its family. The structure carries the pyridinium backbone that tends to irritate eyes and the respiratory tract if handled carelessly. The trifluoromethanesulfonate part brings concerns about corrosivity; nobody enjoys discovering what it can do to skin or lungs the hard way.
Personal Safety Starts with Proper PPE
No one steps up to the bench without a plan. Nitrile gloves protect better than old-school latex against many modern organic reagents. Safety goggles knock down the risk of splash injuries. Cotton lab coats help keep your everyday clothes clean and form a first line of defense in case your hands slip or a container breaks. Closed-toe shoes should stay on every single time—one dropped vial teaches that lesson fast.
One thing I’ve learned the hard way: skip cloth masks or thin surgical masks. They won’t block fine dust or fumes like a certified respirator. Even though N-Ethylpyridinium Trifluoromethanesulfonate usually comes as a crystalline solid, weighing or transferring generates dust, so fume hoods earn their keep here. The best labs I’ve worked in treat open bench weighing of such salts as asking for trouble. Hood sash down, warning light on, and every hand movement planned—this makes a world of difference.
Safe Storage & Handling
This salt absorbs moisture from the air before you realize it. If left out, the jar clumps up, and you can expect unpredictable behavior during the next reaction. Tight seals, desiccators, and sturdy containers help keep the reagent dry and safe. I always label containers with not just the name, but the date received and opened—you never want to guess about shelf life after a few months.
Glass containers work best; certain plastics grow brittle after spending seasons near reactive triflates. If the bottle falls, cleaning up broken glass while avoiding contact with the chemical adds another unwanted hazard. Small aliquots make sense for high-use labs so fewer hands touch the main container, cutting down on contamination and spills.
Spill Response and Waste Disposal
I’ve seen spills minor and major over my career. Quick response matters far more than perfection. Dustpan and brush work only if both are non-sparking and reserved for chemical spills. Never sweep spilled powder with your bare hands or use the communal house broom—standard janitorial supplies aren’t meant for specialty chemicals. Dampen a towel with dilute sodium bicarbonate to absorb powder without spreading it into the air.
Collected waste stays in a clearly labeled bag or container, then heads to the hazardous waste bin for professional disposal. Dumping chemicals like these down the drain poisons water supplies and ignores environmental duty. Lab managers who set up regular waste pickups and clear labeling systems take the burden off individual staff and keep everyone safer.
Culture of Safety Counts
Newcomers pick up habits from those around. Veteran labmates show good technique with every transfer, every cap replaced, and every label written. I’ve listened to too many stories about near-misses to forget the lessons: attention fades fast when pressure runs high or someone feels rushed. Morning safety checks and regular refreshers shape healthy routines that protect more than just individual workers—they carry forward a culture where everyone goes home unharmed.
Good lab practice won’t eliminate every risk, but thoughtful changes make a tough job safer for everyone. Those steps aren’t just smart—they show respect for the work and people in the lab.
Understanding Solubility with a Practical Lens
Lab professionals often come across compounds like N-Ethylpyridinium Trifluoromethanesulfonate and need to decide fast whether it mixes best with water or organic options. This isn’t a topic for idle speculation—getting it wrong sometimes means botched experiments or wasted budgets. Take it from someone who’s rinsed more glassware after failed attempts than I care to admit: knowing your solvent saves time and resources. Solubility shapes how chemists set up reactions, run purifications, and plan proper workflows. It pays to take the subject seriously.
The Chemical Makeup: Clues from Structure
N-Ethylpyridinium trifluoromethanesulfonate offers hints right in its name. Chemists see the “pyridinium” and picture a positively charged ring, friendly with polar environments. Add in the “trifluoromethanesulfonate” part—full of electronegative fluorines—and the picture gets clearer: it tends toward ionic behavior, a solid sign it likes water.
Pour some of this chemical into a beaker of water and stir—the result shows notable solubility, unlike with typical apolar solvents. Solutions get clear quickly, an outcome anyone prepping samples for NMR or electrochemistry notices and appreciates. Compared to big non-polar molecules, ionic salts like this one mesh easily with water because each part finds a good fit. Looking at published solubility tables, the trend holds: salts with similar ionic character generally dissolve in water, a result that shows up again and again in catalogs and technical sheets from chemical suppliers.
Organic Solvents: A Mixed Bag
Drop this compound into acetone or methanol, and it tends to dissolve—a trait that comes in handy for those who need flexibility in their work. Working in pure organic solvents like ether or hexane delivers disappointing results. The strong charge on both pieces of the salt pulls toward polar liquids, and less-polar organic phases just can’t offer those same attractive forces.
I’ve tried dissolving related salts in varied solvents during synthesis projects—polar solvents nearly always win. For labs stuck with greener solvents due to regulation or budget, this might narrow choices, but at least polar options remain plentiful. Methanol and ethanol both work well, and those worried about toxicity look to acetonitrile, which usually pulls its weight in terms of solubility and manageable waste protocols. These facts line up with what chemical safety data sheets and peer-reviewed references report.
Why It Matters Beyond the Lab Bench
This solubility question crosses over from the laboratory to industry, where time and dollars rest on practical solvent use. Soluble salts get used in catalysis, battery research, and drug development, fields that can’t tolerate unpredictable behavior. Imagine trying to scale up a process, only to watch a crucial salt settle out because the solvent choice didn’t fit. Companies need consistency as much as speed, and the wrong mixture means both suffer.
Training chemists in solvent selection can help companies avoid such mistakes. Many organizations now keep solvent selection guides posted in labs or shared in digital form, which cuts down on missteps—something I’ve personally seen drop failed reaction rates in academic and commercial settings. Better yet, encouraging open dialogue about solvent safety, cost, and sustainability builds a smart, collaborative culture.
Looking at Solutions to Solvent Selection Challenges
Investing in better solvent mixtures saves money and risky waste disposal. Easy access to vendor data sheets, real solubility numbers from test runs, and updated safety advice give chemists the confidence to make informed decisions. The field benefits from collaboration with environmental health officers, who keep tabs on which solvents need phasing out for safety reasons, pointing labs toward effective, yet less hazardous options. By treating solvent use as a team effort, labs—both academic and industrial—stand a better chance of staying productive and safe.