N-Hexyl-N-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide: A Commentary
Historical Development
Looking back, the science community saw a real push for safer, more stable electrolytes for batteries through the 1990s and early 2000s. Ionic liquids captured the attention of researchers hunting for non-volatile, non-flammable alternatives to old-school organic solvents. Pyrrolidinium-based ionic liquids like N-Hexyl-N-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide sprang up as chemists got better at tweaking side chains and pairing them with big, fluorinated anions. Labs in Japan and Europe spent years mixing and matching nitrogen-heterocyclic cations with new anions, setting down a recipe for customizable properties. One thing stands out about this compound's entry onto the scene: chemists aren’t just guessing anymore. They chase real improvements—lower toxicity, wider electrochemical windows, and stabler performance across broader temperature ranges—reflecting genuine advances for energy storage and processing industries.
Product Overview
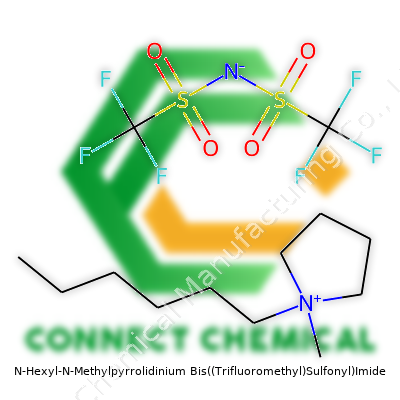
The compound often comes as a clear or pale yellow liquid, hiding within a viscous bottle. Researchers know its structure: a five-membered pyrrolidinium ring, a hexyl group stretching out, a methyl tagging along, and the big bis((trifluoromethyl)sulfonyl)imide counterion bringing serious heft and fluorine power. Folks in labs call it [HMpyr][TFSI] or C14H24F6N2O4S2. The heavy fluorination in the anion boosts chemical and thermal stability. It's become more common in academic supply catalogues, showing up as both a specialty item for battery research and as a reference compound for new ionic liquid blends. The cost keeps it mostly in research hands, but the trend tilts toward larger-scale adoption as purification trails improve.
Physical & Chemical Properties
Pouring out of the vial, the liquid feels slick with low vapor pressure and no obvious odor. Thermal stability means it stays put and doesn’t evaporate easy, even above 200°C. Density lands close to 1.3–1.4 g/cm³; viscosity runs higher than water, which sometimes makes handling in microfluidics a challenge. The wide electrochemical window—often above 4.5 volts—proves the key selling point for energy storage uses. Most researchers appreciate its hydrophobicity and resistance against water uptake, a direct result of the TFSI anion’s big fluorinated arms. Solubility runs high for many organic compounds but it barely mixes with water, a perk when leaks or accidental spills happen. You can’t ignore the low flammability—this compound scoffs at the spark-and-flash risks old electrolytes face.
Technical Specifications & Labeling
Suppliers ship this ionic liquid under strict labeling, including CAS 868675-68-7, molecular weight around 480.47 g/mol, chemical purity often listed at 98% or above. MSDS documents call out all the right warnings: strong chemical gloves, splash goggles, plenty of ventilation. Labeling customs in the industry stress not just purity, but water content—researchers want moisture levels under 100 ppm if possible. Each bottle arrives vacuum-sealed, sometimes blanketed under nitrogen, reflecting a decade of research into stabilization and storage. Barcode tracking brings traceability to every batch, a big leap forward from the days of relabeling jars in the cold room back corner.
Preparation Method
Getting pure N-Hexyl-N-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide on the bench relies on a careful two-step process. Chemists set up a quaternization reaction, starting from N-methylpyrrolidine and hexyl halide, using controlled heating to keep side reactions at bay. After forming the pyrrolidinium halide salt, the next step swaps the halide for TFSI through metathesis, mixing with lithium bis((trifluoromethyl)sulfonyl)imide in water or acetonitrile. Washing, drying, and repeated solvent removal pulls out the last traces of water and inorganic salts. The final cut needs vacuum drying, sometimes for a day or more, before the product wins approval for electrochemical tests. This prep shows how much the field values true reproducibility—good product beats quick product in ionic liquid chemistry.
Chemical Reactions & Modifications
The fun with [HMpyr][TFSI] starts when researchers mess with its alkyl chains or test new anions for even better properties. Swapping the hexyl group for something longer or shorter changes viscosity and melting point; flipping in a bulkier side chain can tune solubility or ion mobility. The nitrogen center lets in a range of substituents, each rewriting how the whole ionic molecule stacks and conducts. On the anion side, new variants like FSI (fluorosulfonyl imide) open up different conductivity and stability profiles—chemists track even small tweaks in electrochemical cells with unforgiving precision. Some push the compound into polymer matrices, while battery labs graft it onto functionalized films, chasing better cycling and safety. The chemical flexibility of this family of ionic liquids drives the growing number of patents and publications.
Synonyms & Product Names
Across industry and academia, the most common synonyms mirror the mouthful chemical name: [HMpyr][TFSI], N-hexyl-N-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide, and 1-hexyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide. Some catalogs shorten things further, listing simply “Pyrrolidinium TFSI” or “HMpyr TFSI.” Tracking down technical literature means checking for these aliases, especially in cross-language journals. Commercial sources keep to the IUPAC style to avoid confusion. In real lab talk, researchers just point to “the hexyl pyrroli TFSI” as a kind of shorthand, a small marker of how the compound has found a place in day-to-day research.
Safety & Operational Standards
No one takes safety for granted with ionics like this. Researchers gear up with nitrile gloves, full goggles, and work under fume hoods even as toxicity risks run lower than plenty of legacy solvents. TFSI-based liquids push aside the old volatility hazards, but they don’t let operators ignore chemical hygiene. Inhalation hazards don’t quite vanish—they just drop down compared to chloride or fluorinated organic counterparts. Electrochemical cells get isolated in glove boxes, not only for safety but also to preserve purity. Cleaning spills calls for absorbent pads and plenty of ventilation; the compound’s strong fluorination means special care in waste disposal, steering clear of halogen emission in incinerators. Regulatory bodies continue updating their standards as more labs switch to these ionic liquids, but the culture moves faster than bureaucracy.
Application Area
[HMpyr][TFSI] earns its reputation in energy storage, powering test cells for next-generation lithium metal, sodium ion, and hybrid batteries. Lab groups chasing higher voltage windows or safer solid-state designs lean into the compound’s resilience; it shrugs off the heat and doesn’t crash on voltage spikes. Far from just batteries, it blends into double-layer supercapacitors, organic redox flow cells, and as specialty solvents for tricky organic syntheses. Its low volatility lets folks dream up safer electroplating baths and more predictable lubrication in wear-intensive gear. Even pharmaceutical researchers test its potential in drug solubilization and analysis, hunting for solvents with fewer biological side effects. Universities and corporate R&D labs compete to patent new uses, but all appreciate that the compound soaks up energy without giving out dangerous fumes or corroding their gear.
Research & Development
Over the last decade, publications on pyrrolidinium ionic liquids exploded, with [HMpyr][TFSI] earning hundreds of citations from electrochemists, material scientists, and chemical engineers. The move to solid-state and lithium metal batteries forced labs to find stable, high-conductivity electrolytes—this compound often sets the baseline for tests. Collaborations between academia and industry push the envelope, combining it with advanced polymers and nanomaterials. Graduate students track every tweak in conductivity, viscosity, and voltage breakdown thresholds, reporting their findings through open access repositories. Markers of real progress include the emergence of scalable syntheses, improved purification trains, and databases tracking compatibility with sensitive electrodes. The speed of innovation in this space means tomorrow’s breakthroughs could stem from yet another modification to this molecule’s backbone.
Toxicity Research
Scientists never turn a blind eye to toxicity concerns. Studies using brine shrimp, zebrafish, and mice check for acute toxicity and bioaccumulation risks. So far, results indicate [HMpyr][TFSI] runs lower on acute toxicity charts than many older organic solvents or imidazolium-based relatives. Chronic exposure effects remain under scrutiny; some animal models do reveal mild enzyme inhibition and metabolic shifts. Environmental chemists screen for breakdown products in water and soil, noting the compound’s persistence thanks to its fluorinated tail. Calls grow louder for standardized, wider studies before large-scale deployment in consumer-facing products. Factories moving to scale up production face tough questions on emissions and containment, and those who remember the PFAS controversies push for closed-loop recycling and tough waste tracking.
Future Prospects
Keeping an eye on where [HMpyr][TFSI] goes next means tracking both supply chain improvements and the race for better battery builds. Next-generation energy storage—solid-state lithium, sodium, and magnesium systems—need tougher, less flammable electrolytes. This compound checks off the wish list: high voltage stability, minimal toxicity, and stubborn resistance to both water and heat. Scaling up remains the bottleneck as production costs and quality consistency lag behind the best organic solvents. Researchers push for greener synthesis methods, fewer halogen waste streams, and biodegradable alternatives. The future of this compound hangs on its ability to adapt—to new anion combinations, to tighter environmental scrutiny, and to a world asking tough questions about every molecule’s cradle-to-grave impact. The drive for safer and more sustainable technologies means N-Hexyl-N-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide stays in the research spotlight, and its next chapter gets written in the world’s battery plants, university labs, and regulatory debate rooms alike.
Insight Into a Modern Ionic Liquid
N-Hexyl-N-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide—quite a mouthful for a compound that’s found a place in some cutting-edge technologies. In the world of chemistry labs and industrial innovation, people often chase performance. From what I’ve seen, this ionic liquid delivers on both stability and flexibility. I’ve worked alongside researchers testing new batteries and folks scaling up processes that demand both safety and purity, and this is one of those tools that quietly shapes the future.
Powering New Age Batteries
Talk to anyone searching for safer, longer-lasting batteries, and you’ll probably hear about ionic liquids. N-Hexyl-N-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide, or Pyrrolidinium-TFSI, is drawing attention for its thermal stability and wide voltage window. Lithium-ion battery teams are using it instead of traditional solvents because it stands up to heat and doesn’t catch fire easily. In high-voltage lithium-metal and lithium-sulfur batteries, Pyrrolidinium-TFSI helps reduce side reactions. This means more charge cycles, less swelling, and safer operation.
Solid-state battery development also leans on ionic liquids like this one to enhance ion mobility in the polymer electrolyte. While legacy electrolytes restrict battery designers, Pyrrolidinium-TFSI opens the door for flexible forms, including wearable tech and next-gen grid storage. Watching these batteries outperform their conventional counterparts is a reminder that progress usually comes from details like the choice of salt and solvent—things many folks outside the field rarely see.
Electroplating and Metal Processing
The move to greener electroplating started with government pressure, but the best changes come from inside the shop. Throw away those cyanide-based solutions; Pyrrolidinium-TFSI supports clean metal deposition with less environmental hazard. Aircraft and electronics manufacturers aiming to cut toxic effluents switch to these ionic liquids for aluminum and rare metal coatings. In my experience, adoption hinges on reliable supply lines and cost, but the shift is happening as environmental rules tighten. Factories want processes that don’t require an army of hazmat suits just to keep the place legal.
Industrial Lubrication
Machinery faces tremendous stress in demanding environments, whether drilling deep under the sea or running turbines in a desert. Ionic liquids like Pyrrolidinium-TFSI, with their non-flammability and outstanding chemical resistance, offer a solution for gear systems that fail under classic oils. The stuff stays liquid and maintains performance over a wide temperature range, which prolongs the life of parts and cuts maintenance. These are differences that show up big on the balance sheet. Keeping a rig running longer with fewer shutdowns is a goal every engineer understands.
Other Specialized Uses
Researchers also test this ionic liquid in gas separations, dye-sensitized solar cells, and even sensors where toxic alternatives don’t fit. Its low volatility makes it attractive for enclosed or sensitive environments. Every so often, I meet someone mixing custom formulations for high-performance liquid chromatography or lab-scale synthesis—the compound keeps popping up as a go-to solvent for reactions that stall out with anything less robust.
Looking Ahead
Adoption isn’t without hurdles. Price and large-scale production require creative solutions, like new synthesis pathways or recovery processes. Industry insiders are pooling data on recycling and lifecycle analysis, hoping to demonstrate not just technical performance but also responsible sourcing and disposal.
The push for safer, sustainable technology isn’t slowing down. From the batteries in our cars to the coatings on our electronics, N-Hexyl-N-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide is proving its worth as both a high performer and a piece of the environmental puzzle.
Understanding the Basics
Physical and chemical properties aren’t just words from a dusty high school textbook. They shape the world around us, from the salt in our kitchens to the medicine we trust. When handling any compound, folks often start with basics like color, smell, and feel. For instance, table salt comes as a white, granular solid, dissolves easily in water, and carries a sharp, briny taste. That’s practical information anyone can relate to, and it sets the tone for how we deal with chemicals across the board.
Looking Closer at Physical Properties
Everybody who spends time in a lab notices how melting and boiling points set boundaries for safe handling. A high melting point means a compound won’t liquefy in your hand. Water boils at 100°C, which already tells us it can only take so much heat before things change. Density also decides if an object floats or sinks — oil and water won’t mix, and their different densities make a salad dressing split in a bottle even before you pour it. Sometimes you see this in more unusual places. I once watched a colleague try to combine two powders for a project, only for one to keep floating to the top of our beaker. It wasn’t magic — just density at work.
Chemical Properties in Action
Most of the drama in chemistry comes from chemical properties. How something reacts when mixed with air, water, acids, or bases changes everything. A big reason that household bleach says “do not mix with ammonia” is because a nasty gas forms in their reaction. Stability matters, too. An unstable compound might break down over time or even explode with a simple spark — think about the warnings on old cans of polyurethane finishes. This isn’t just caution for the sake of it. A chemical’s flammability means someone working in a garage has to keep rags away from open flames.
Learning from Experience
During college research, carelessness with simple properties once led me to stain my favorite jeans with a bright purple compound. Its solubility took me by surprise — I’d expected it to sit there, but it dissolved in water, spreading further than I’d planned. That moment drove home that ignoring the basics — solubility, volatility, how easily something can evaporate — wrecks good work. In manufacturing, ignoring these details costs money and sometimes safety. For pharmaceuticals, chemical purity and reactivity changes how effective a drug ends up inside the body.
Practical Ways to Address Issues
Grasping these properties pushes us toward better habits. Labeling storage containers by hazard level and keeping incompatible chemicals apart in everyday workplaces saves time and health. In agriculture, understanding soil chemistry helps farmers pick the right fertilizers, steering away from reactions that waste precious nutrients. In household settings, folks can reduce risk by storing cleaning products away from heat and checking expiration dates. For those mixing homemade cleaners, a quick read through a material safety data sheet brings practical advice in plain language.
Science in Real Life
Paying attention to the physical and chemical properties of compounds isn’t theory buzzing over scientists’ heads. It’s a part of building safer homes, crafting better medicines, and making smarter choices in everyday life. The details might start in the lab, but the lessons end up in pantries, workshops, and fields across the world.
Chemicals in the Lab: Why Real Precautions Matter
I’ve worked in enough labs to know that even chemicals that don’t have skull-and-crossbones labels can catch people off guard. Names like N-Hexyl-N-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide can make your eyes glaze over, but ask anyone who’s handled new-generation ionic liquids, and you’ll hear the same thing: don’t take shortcuts. On paper, this compound seems promising, with uses in batteries, electrochemistry, and advanced material science. The catch is, its safety data sheet comes with warnings that are easy to overlook, especially if you start treating it as “safe by default.”
Common Sense and Chemicals: What’s Actually at Risk
Just because something isn’t volatile or doesn’t stink up the room doesn’t mean it won’t mess with your health. I’ve seen some folks get too familiar with ionic liquids, only to end up with unexplained skin issues after a few days of use. N-Hexyl-N-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide is lipophilic, and its toxicity profile isn’t fully mapped out. The lack of acute toxicity reports isn’t the same as a green light for careless handling. Several research studies suggest ionic liquids in this family can cause burns, eye irritation, and potential long-term health problems — even though they don’t always hit you with a strong odor or immediate reaction.
Handling: Don’t Leave It to Chance
In my experience, gloves are non-negotiable. Go for nitrile over latex — these chemicals can sneak through latex surprisingly fast. Wearing splash goggles has saved me more than once, especially during weighing and transfers. Labs with decent airflow or fume hoods give a better shot at keeping the air clean if spills or evaporation happen. I make it a habit to check the chemical’s SDS before every new batch. Just one missed update can mean missing information about new hazards.
Spills might tempt the “paper-towel-and-hope” approach. Don’t. This stuff can stay on surfaces and create exposure risks for everyone using the space later. Absorb with appropriate material — not your old cotton towels — and bag the waste for authorized disposal. Handwashing isn’t optional after glove removal, even if the gloves look clean. Many of us have stories about colleagues who wiped sweat or rubbed their eyes after “just a quick clean-up,” and regretted it.
Storage and Training: A Team Effort
Keeping N-Hexyl-N-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide in a tightly sealed container, away from acids and oxidizers, avoids surprises. Stashing it on a high shelf or beside incompatible chemicals increases accident risk. Each new student or colleague should sit down for a real talk about why PPE isn’t just box-ticking, even for chemicals that don’t seem dangerous straight away.
Adhering to protocols isn’t just about personal safety — it’s about the whole team’s wellbeing. I’ve seen productivity drop for days after a preventable incident. Time spent on training and putting the right practices in place keeps things running smoothly and protects everyone in the lab. Reading recent research is worth it, too, since the field keeps evolving and new findings on toxicity sometimes change the safety landscape fast.
Taking Safety to Heart
Nobody wants to end up as the reason new safety rules get posted on the wall. My own close calls in the lab taught me that respected chemicals can have hidden risks. Staying informed, wearing the right gear, and treating every use with respect keeps both you and your team out of trouble.
Why Shelf Life Actually Matters
No one likes the surprise of finding a can in the pantry that expired a year ago, even less so when it goes unnoticed until it’s opened. Shelf life isn’t just a technical number on a label. That date helps consumers know how long a product keeps its quality and, more importantly, stays safe to use. If you’ve ever had bread that turned green before the “best by” date, you know that storage conditions can ruin things faster than printed labels suggest.
Common Shelf Life for Everyday Products
Most packaged foods carry a range between six months and two years on their labels. Take dry pasta as an example; kept dry and out of the sun, it might last three years. Canned beans make it even further, powering through two, even five, years if kept at a steady indoor temperature. The difference lies in how sensitive each product is to air, moisture, and heat.
For vitamins and supplements, the windows tend to shrink. Sensitive ingredients break down under humidity or high temps, so letting a vitamin bottle sit on your car dashboard can destroy its value in days, not months.
Storage: Not Just a Suggestion
Manufacturers print their best advice straight onto the label. “Store in a cool, dry place” isn’t just legal filler. Cool temperatures slow chemical changes and keep spoilage away. Humidity invites mold and speeds up rust or rot, depending on what you’re storing. Personal experience backs this up—leave flour in a steamy kitchen cabinet, and sooner or later bugs will find it.
Direct sunlight causes just as much havoc. Transparent containers let in light that beats down on oils or packaged snacks, making them taste stale long before a sell-by date. Even hardware reacts. Anyone who’s stored batteries on a bathroom shelf knows that moisture means corrosion, and corrosion means wasted money.
Science Behind the Numbers
Storage recommendations come straight from food scientists and quality control experts, not marketing teams. Labs stress-test products, exposing them to extreme temperatures, bright light, or humid air. What gets measured is the point where safety goes down or flavors change. Reports from the Food and Drug Administration regularly show that mishandled products cause issues, not recipes or formulas.
The U.S. Department of Agriculture backs up this approach. Staples such as rice last decades in airtight containers, but only if stored under steady, cool conditions. In contrast, dairy and meats demand refrigeration below 40°F—one slip, and dangerous bacteria grow in hours. Remembering this can spare you from a nasty stomach ache or, in worst cases, more serious health risks.
Simple Steps That Stretch Shelf Life
Paying attention to where and how items are stored can mean real results. Move dry goods to airtight containers; don’t trust paper boxes or bags. Set pantry shelves away from sunlight and heat sources. Check freezers or fridges occasionally for cold spots and leaks. Even rotating newly bought stock to the back can prevent wasted food and money.
Not all advice comes from scientists or labels, though. Family traditions—like never leaving tomatoes in the fridge or always storing potatoes in the dark—have roots in generations of observation. Modern research often just confirms what people learned the hard way.
Takeaway: Practical Storage Pays Off
Paying attention to shelf life and storage conditions has saved my household more money than any coupon ever could. Nobody wins by gambling with old food or ignoring safe storage tips. It’s easier to build good habits, scan a few labels, and make small changes than to deal with the aftermath of spoiled food or wasted goods.
Why Purity Matters in Chemical Purchases
Seeking purity information isn’t just about checking a box on a spec sheet. Purity directly impacts how well a chemical performs, how safe it is to handle, and how suitable it is for a particular process. Take lab reagents, for example. If a product’s purity hovers around 98%, you may think you’re set for routine analysis, but a trace of heavy metals or moisture can derail experiments or gum up industrial processes. In food and pharmaceutical settings, contaminants carry even greater consequences, with the risk of patient exposure or product recalls. Relying on a Certificate of Analysis (COA) from a reputable supplier forms a critical step in verifying claims. The COA should show batch-specific data—actual percentages, not just a label.
MSDS: The Undisputed Guide to Safety
Every chemical shipped, handled, or used anywhere in the supply chain ties back to its Material Safety Data Sheet (MSDS), sometimes called SDS under GHS labeling. Think of the MSDS as more than a bureaucratic formality. This document spells out physical and chemical properties, health hazards, environmental risks, first aid, firefighting instructions, and safe storage conditions. Anyone dealing with chemicals, from seasoned lab techs to warehouse staff to logistics handlers, needs easy access to up-to-date MSDS documents. Outdated safety instructions, or suppliers who send incomplete instructions, raise serious red flags. The MSDS is a living resource: OSHA requirements in the US, CLP in the EU, and equivalents elsewhere keep evolving. Workers’ right-to-know laws make distribution of the MSDS not optional, but a legal and moral obligation.
Packaging Sizes: Options Aren’t Just About Convenience
Choosing the correct packaging size directly influences cost, safety, waste, and ease of use. Small batches of high-purity chemicals often come in amber glass bottles to shield contents from light and moisture. Technicians handling active pharmaceutical ingredients commonly need packs as small as 1-gram aliquots for dose accuracy. Bulk buyers may receive drums, carboys, or even IBC totes for larger consumption. Improper packaging, or defaulting to the wrong size, can ramp up user error, force rushed re-packaging, or drive up transportation fees. For volatile or sensitive substances, careful packaging preserves both safety and chemical stability.
Supplier Reputation and Verifiable Information
A trustworthy supplier stands out by providing transparent purity specs, batch-specific COAs, instantly available MSDS documents, and smart packaging choices. It pays to check for third-party certifications—ISO 9001 for quality management is a good marker—and ask for references or product samples. Suppliers open to independent audits show they understand that documentation alone does not build trust. Open channels for tech support and after-sales consultation can reveal how tuned-in a vendor is to end-user needs and regulatory trends.
Pushing for Practical Solutions
Better purity tracking starts with direct communication between buyers and suppliers. Always request updated COAs for every batch and store digital backups. Keep a central digital MSDS repository, audited regularly, so everyone from procurement to emergency responders stays informed. Collaborate with suppliers about your workflow for a smarter packaging fit. Train staff regularly on chemical safety, including MSDS content and best practices for handling, not just on paper but through practical drills.
The difference between a safe, productive operation and a costly misstep often rests on clear information and smart choices about purity, safety data, and packaging. Real accountability from suppliers, and vigilance from buyers, protects both people and business.