N-Methylimidazolium Dihydrogen Phosphate: Impact, Development, and Discovery
Historical Development
Scientists have explored ionic liquids for decades, chasing safer and more efficient alternatives to classic solvents. N-Methylimidazolium Dihydrogen Phosphate caught attention after the early 2000s, as researchers noticed its strong ionic character and unique compatibility for catalysis, extraction, and electrochemical work. Chemists wanted low-volatility compounds for green chemistry, and this salt-like liquid, with its stable imidazolium core, filled a gap many had struggled with. I remember seeing the first academic papers rolling out, probably inspired by earlier generations of imidazolium cations, which themselves rose in the late twentieth century through the pioneering work of chemists like Wilkes and Zaworotko. This phosphate variant introduced enhanced acid-base interplay, pulling in teams hoping for better manipulated chemical environments and new tech for battery research, biomass processing, or even pharmaceuticals.
Product Overview
N-Methylimidazolium Dihydrogen Phosphate steps beyond conventional salts and liquids. Unlike legacy organic solvents, it offers negligible vapor pressure and elevated ionic conductivity. Its emergence met strong industrial demand for solvents that mitigate flammability and air emissions. Its unique molecular framework—a methylated imidazole ring attached to a dihydrogen phosphate anion—sets it apart in specialized chemical processes. Handling the bottle, you feel faintly viscous liquid, almost syrupy, sometimes appearing as a colorless or pale yellow solution, transmitting both a modern laboratory feel and a clear utility in practical, lab-scale and industrial applications.
Physical & Chemical Properties
In terms of basics, this compound tends to draw water—hydrophilic by nature. Most samples arrive as viscous liquid or low-melting solid. The ionic bonds grant thermal stability beyond typical solvents. It handles harsh acidic or basic conditions and tolerates high temperatures, often with decomposition only past 200°C. Electrical conductivity remains high for a salt, which suits it to electrochemical experiments. Its modest molecular weight (usually below 250 g/mol) makes it a preferred candidate for low-toxicity laboratory use. A strong salt-like taste underscores the low volatility, though you'd never want to risk direct exposure. From my own time working in a synthesis lab, storage never required fume hoods for evaporation, a benefit over most classic solvents.
Technical Specifications & Labeling
Manufacturers commonly ship it with assay percentages noted above 98%, and explicit impurities, such as chloride, sulfate, or free phosphoric acid, kept below 0.1%. Labels prominently state "Keep container tightly closed," and hazard labeling incorporates GHS pictograms for irritant and, occasionally, corrosive hazards. Documentation must specify batch number, recommended storage (cool, dry location), and guidance on safe transfer, since contact with skin or eyes may trigger irritation. REACH and local chemical inventories demand listing for workplace safety compliance, so each delivery comes with thorough Safety Data Sheet (SDS) detail, much like any other potent laboratory reagent with potential for skin and mucous membrane irritation and moderate environmental impact.
Preparation Method
The synthetic approach usually starts with methylimidazole reacted with phosphoric acid, measured out to yield precise stoichiometry for the dihydrogen phosphate. Vigorous stirring, often under gentle heat, speeds reaction, though cooling becomes important to prevent byproduct formation. Purification relies on removal of excess acid and organic starting material, usually by vacuum distillation and repeated washing with ethanol or water. Researchers learned to dial in yields by careful titration, always tracking pH and color change as the best signposts for reaction advancement. If you lack careful pH control, chances for phosphate overloading or unwanted side reactions jump, a situation I once faced and resolved through benchtop titration and close collaboration with analytical chemists.
Chemical Reactions & Modifications
N-Methylimidazolium Dihydrogen Phosphate behaves as a mild acid in water, pairing with bases for salt metathesis or controlled pH adjustments in sensitive chemical syntheses. In catalytic reactions, it routinely outperforms common organic acids due to strong ionic environment and minimal self-degradation. It won't decompose under common lab lighting or air contact, though it can hydrolyze a few water-sensitive organics. Adding alkali metals kicks off ion exchange reactions, producing alternative phosphate compounds or hybrid catalysts. For more advanced uses, chemists attach functional groups to the imidazolio ring, tuning solubility or increasing reactivity—modification strategies that extend applications in pharmaceutical or materials science circles, something many researchers are eager to explore further.
Synonyms & Product Names
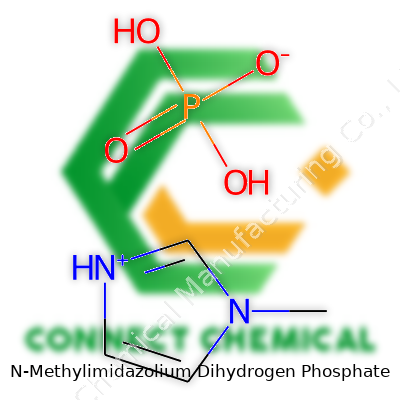
On purchase orders and academic papers, you see a mix of synonyms such as 1-Methylimidazolium Dihydrogen Phosphate or abbreviated names like [Hmim][H2PO4]. Some catalogs opt for "Imidazole, 1-methyl-, dihydrogen phosphate," catering to systematic naming conventions. These names signal slightly different naming philosophies across chemical suppliers. Still, the chemical fingerprint—methyl group attached to nitrogen at the first position, paired with the iconic dihydrogen phosphate anion—appears as a throughline across labels, safety documentation, and regulatory filings.
Safety & Operational Standards
Safety training forms the first barrier against mishap with this product. Lab coats, nitrile gloves, and splash goggles reduce risk from skin or eye contact. If spilled, cleanup calls for absorbent materials and gentle neutralization—phosphate ions wash down drains only with copious dilution. Some workers report mild irritation after repeated exposure by skin, though inhalation risk stays low due to its low volatility. Regulations urge rigorous labeling, closed-system transfers, and chemical hygiene practices, mostly to keep even minor exposures under control. Disposal aligns with standard phosphate waste procedures, with clear restrictions on large-volume disposal to avoid water treatment plant overload. As a chemical worker, you value routine and vigilance, lessons driven home through real near-miss events and shared anecdotes in yearly safety refreshers.
Application Area
Industries apply this compound in batteries, metal plating, green chemistry, and select pharmaceutical syntheses. Electrochemical cells run better using it as solvent or ionic conductor, boosting ion exchange in fuel cell membranes. Catalysis benefits from its acid-rich profile—chemists push forward reactions without needing strong mineral acids, which helps reduce both hazards and waste. In textile processing, the compound gets applied as a dye solvent, where its ionic nature improves color fixation. For biomass conversion, researchers discovered it dissolves cellulose, allowing for gentler, more sustainable material processing. My academic colleagues in biofuel development shared stories of using it to unlock stubborn plant matter, opening up routes to new fuels and materials with smaller environmental footprints.
Research & Development
Current research dives into renewable chemical processes, latching onto ionic liquids as essential tools for sustainable production. Study after study weighs N-Methylimidazolium Dihydrogen Phosphate’s catalytic abilities, especially at low toxicity levels, versus classic mineral acids. The academic world now explores its place in enzymatic reactions—one area seeing heightened funding due to biotech growth and the search for more mild, tunable chemistry. From a firsthand view, university labs run experiments adjusting the methyl group or swapping phosphate variants, chasing improved conductivity, solubility in non-aqueous media, and lower manufacturing costs. Pilot projects focus on scaling up green manufacturing without burning through resources or creating new pollutant headaches, feeding a persistent drive for greener, smarter chemistry anchored in practical, reproducible lab work.
Toxicity Research
Toxicologists feel fairly confident about this compound’s lower toxicity compared to legacy solvents. Animal studies track moderate oral and dermal effects—outcomes typically more benign than imidazolium salts with halide or perfluorinated anions. Cell assays show little long-term genetic impact at ambient levels, and environmental assessments mostly highlight phosphate enrichment as the main concern, something water treatment and process design address. My old laboratory ran routine tests on runoff, double-checking aquatic life impacts, which sparked promising conversations about phosphate cycle management. Without persistent organic pollutants or cumulative metal residues, discharge concerns feel manageable, though no one suggests careless handling. Overall, real-world toxicity stays low, as long as lab procedure and disposal standards get followed.
Future Prospects
The outlook for N-Methylimidazolium Dihydrogen Phosphate stays bright across research and industry. Green chemistry momentum beckons for more ionic liquid adoption—and this compound ranks as a forerunner for applications demanding both stability and tunable acidity. Battery developers want new electrolyte systems for higher efficiency and safety. Biotech pushes ahead with enzyme-compatible solvents, where this phosphate salt repeatedly rises to the top of candidate lists. Manufacturing scale-up brings the final challenge: lowering synthesis costs and improving recycling. New partnerships between academia and industry search for improved reaction design and more robust production, helped along by fast, pragmatic testing in pilot and full-scale plants. Plenty of opportunity emerges for those ready to invest in greener, safer, and more effective chemicals, moving industry away from twentieth-century hazards and toward genuinely cleaner production for the decades to come.
What Stands Out About N-Methylimidazolium Dihydrogen Phosphate?
N-Methylimidazolium Dihydrogen Phosphate isn’t a chemical you meet every day outside a research lab, but it carries a lot of weight in several important industries. As a chemist, I’ve occasionally run across compounds that show up quietly, doing their job behind the scenes. This one fits that mold. Its appeal comes from a unique combination of stability, tunable properties, and compatibility with green chemistry. These traits set it apart from many traditional solvents and catalysts that burden both users and the environment.
Helping Clean Up Industrial Chemistry
Industries look for safer, more sustainable chemicals. Traditional solvents cause plenty of headaches. They often evaporate quickly or produce toxic byproducts. N-Methylimidazolium-based salts, including this phosphate, replace more hazardous solvents because they hardly evaporate, cause less pollution, and don’t ignite as easily as organic alternatives. I remember preparing samples with volatile solvents and worrying about fumes. Switching to ionic liquids like this makes the lab safer without sacrificing performance. As a bonus, workers run a lower risk of chronic exposure to toxins.
Catalysis with an Environmental Edge
One of the most interesting uses is in catalysis, which saves energy and keeps reactions running efficiently. In my experience, finding the right catalyst transforms a tedious reaction into an efficient one. N-Methylimidazolium Dihydrogen Phosphate often gets used in transesterification and cellulose processing because it keeps biopolymers dissolved and stable. Factories processing biomass and making biofuels can cut waste using less energy, which sends fewer emissions into the air. Lately, there’s also movement toward using these ionic liquids for plastic upcycling and green polymer synthesis, where speed and selectivity matter just as much as safety.
Batteries and Energy Storage
Modern batteries need stable electrolytes that don’t break down or burst into flames. I’ve followed a few studies out of Asian and European labs where N-Methylimidazolium Dihydrogen Phosphate steps in as a safer alternative to lithium salts in supercapacitors and next-generation batteries. Safer batteries mean fewer fires in phones and cars, and better stability over long charging cycles. The salt’s thermal stability gives designers more freedom to create devices that last longer and perform reliably in hot or cold conditions. This isn’t just about convenience—it actually reduces the long-term cost and risk for users and manufacturers alike.
Room for Improvement
This compound raises the bar for responsible chemistry, but it isn’t perfect. Price slows its broader adoption. Manufacturers still depend on scaling up production for these ionic liquids, which takes money and investment. Open-source data sharing and more robust government support for green chemistry would put this material in more hands. Universities partnering with industry could focus on affordable synthesis routes. Researchers have started making small improvements by tweaking molecular structure so that the salt works in even tougher environments and dissolves cheaper, widely-available materials. Momentum seems to build with every success.
The Bigger Picture
N-Methylimidazolium Dihydrogen Phosphate proves that safer and more effective chemical ingredients can become realistic options, not just “green dreams”. In my own work, shifting focus toward new ionic liquids led to creative problem-solving and smaller environmental footprints. Factories, labs, and battery makers gain new ways to do better work with less risk, and that feels like progress that matters.
Chemical Formula and Structure
N-Methylimidazolium dihydrogen phosphate carries the formula C4H9N2O4P. This comes from its two parts: the N-methylimidazolium cation and the dihydrogen phosphate anion. Chemists build this cation by adding a methyl group to imidazole, a five-membered ring with two nitrogens. Then, add the H2PO4- to bring everything together. At a glance, the molecule looks simple, but this combination brings about some nifty properties that keep popping up in labs and industry projects everywhere.
Why It Matters in Chemistry and Industry
The subject might seem narrow at first, but anyone who’s spent time mixing solutions or searching for stronger electrolytes knows ionic liquids change the game. N-Methylimidazolium dihydrogen phosphate is one of these ionic liquids, and it stands out because it’s less volatile and rarely catches fire compared to traditional organic solvents. Picking the right substances keeps workplaces safer, especially if you’re working in tight academic labs or pilot plants that don’t tolerate accidents.
I recall an experiment years ago, swapping out volatile solvents for ionic liquids in catalysis reactions. The number of fume hood alarms and headache complaints went way down. The choice of phosphate as the counter anion adds acidity and solvating ability, which helps when dissolving metal oxides or tweaking reaction pathways in synthesis.
Real-World Impact and Challenges
Green chemistry demands reliable substitutes for old-school solvents. N-Methylimidazolium dihydrogen phosphate doesn’t evaporate into the air quickly, so the workplace smells cleaner. It often stays liquid at room temperature, so researchers skip complicated heating setups. Many projects aiming for sustainable chemistry pick this compound because it can dissolve everything from cellulose to transition metal salts, opening up recycling or bio-refining options that older solvents wouldn’t allow.
Problems show up, too. Cost has a way of killing great ideas in scale-up. Sourcing high-purity starting materials for these salts costs more than buying cases of plain acetone or toluene, although the savings on ventilation and fire protection balance things out over years. Disposal still needs care: just because a material has low vapor pressure doesn’t mean it disappears without a trace. Left unchecked, phosphates add to eutrophication risks in waterways, so treatment before release makes a difference.
Finding Better Roads Forward
Researchers look for ways to make N-methylimidazolium dihydrogen phosphate cleaner and cheaper. Some new reactors can recycle ionic liquids almost endlessly. Others turn to biobased imidazole synthesis routes to cut down on fossil input. My favorite approach draws from community: share lessons between groups, talk about real mistakes, not just published results, and test these liquids on practical problems, not just high-profile demonstrations.
The formula C4H9N2O4P is just a starting point. The mix of safety, chemistry, and rising sustainability priorities means its story is far from over. As labs and manufacturers put it to work, the knowledge spreads, inching research closer to safer, cleaner, and more resourceful solutions—proof that a shift in the way we build molecules can shape not just reactions, but whole industries.
Understanding the Chemical
N-Methylimidazolium dihydrogen phosphate sounds like something straight from a lab bench, and that's for good reason—it belongs to a family of chemicals called ionic liquids. These compounds often show up in research on green chemistry because they help dissolve tough materials that regular solvents can't handle. Still, just because a chemical helps break down biomass or improve battery performance doesn’t guarantee it gets a clean bill of health.
The Health Question
Every time a new chemical shows promise, safety rises as the first real-world hurdle. Unlike table salt or sugar, you won’t find a long trail of safety data for N-Methylimidazolium dihydrogen phosphate. Academics favor the broader group—imidazolium-based ionic liquids—because they carry a low vapor pressure, which means less stuff goes airborne in a typical use. This property matters since dangers like inhalation become less likely compared to old-school solvents like toluene or acetone.
Toxicity, though, shouldn’t hide behind big words or the excitement of “green” chemistry. A handful of toxicity studies show some imidazolium-based ionic liquids irritate skin and eyes if spilled. In animal studies, other chemicals of this family caused damage at higher concentrations, mostly attacking cells or internal organs with prolonged exposure. For N-Methylimidazolium dihydrogen phosphate specifically, little direct animal or human data exists—so caution makes sense.
Environmental Fate
Any solvent sticking around in water supplies raises hard questions. Imidazolium salts often degrade slower than traditional solvents. Some researchers saw them harm fish and microalgae at concentrations as low as a few milligrams per liter in controlled water studies. If someone spills a large batch, the danger isn’t always obvious in the short term. Instead, these chemicals creep through water and soil, sometimes sticking around long enough to harm sensitive plants or aquatic life.
In my own research assistant days, I watched as solvents got poured down lab drains with little thought, the focus always on the next reaction or paper. Letting new chemicals slip into the environment without proper storage or disposal just repeats old industry mistakes. Such habits don’t fit responsible science.
Regulatory Status and Worker Protection
Right now, global chemical registries like REACH in the European Union don’t single out N-Methylimidazolium dihydrogen phosphate for extra restrictions. That doesn’t mean safety is certain; it just means the paperwork hasn't caught up yet. Labs and small-batch producers often use the precautionary principle—wear gloves, use goggles, and handle this chemical in a fume hood, since irritation and unknown chronic risks linger in the background.
Bringing a new solvent into the plant or classroom asks everyone to treat it with the same respect earned from generations of working with unknowns. Treating every liquid like it’s harmless opens the door to repeat the mistakes that built worldwide trust issues with new technologies and chemicals.
Potential Steps Forward
N-Methylimidazolium dihydrogen phosphate could offer new solutions in green chemistry, battery tech, and more. But skipping routine toxicity and environmental testing sells short both workers and communities living near production sites. Pressure from universities, industrial labs, and green chemistry advocates can move funding toward long-term studies and clear labeling.
Sending chemicals off for third-party safety tests before wide release doesn’t drag innovation backward. It lays the groundwork for trust and real progress. People want safer chemicals, and companies that back research and open data build the strongest reputations in the long run.
Looking After Chemicals—Not Just for the Lab Coat Crowd
Let’s get honest about chemicals and storage. N-Methylimidazolium dihydrogen phosphate comes up in labs, and sometimes in R&D corners of industry, as a handy ionic liquid. It isn’t something you find in kitchen cupboards, but anyone who’s cracked a chemistry textbook gets that smart storage isn’t about paranoia—it’s about avoiding mistakes you can’t take back. My own early confusion around chemicals involved two unmarked bottles, a careless shelf mate, and a hot sunny window. The stench taught me: tiny lapses pile up fast.
The Real Risks and Facts
We live in a world full of safety sheets and data, but experience counts, too. This compound won’t leap across the room to hurt anybody, yet it brings real risks if ignored, especially where moisture and air sneak in. N-Methylimidazolium salts, like many ionic liquids, pull in water if you leave them out. They call this behavior “hygroscopic,” but that dry word underplays what even small humidity can do. Water changes the compound, which can lead to instability or loss of performance. Sometimes you won’t spot the change until a reaction fails or a machine jams. I’ve seen coworkers toss out a whole batch rather than trust a bottle left unsealed.
Looking past water, temperature creates its own problems. Leave the bottle near a window or on a radiator, and slow breakdown starts. Some of the byproducts give off a smell—a good warning, but the damage gets done before the odor arrives. Heat can drive unwanted reactions, making surprises possible in the next round of work.
Where and How to Store It—Making It Simple
Best choice is a cool, dry spot. Room temperature usually keeps things steady, but tight temperature swings spell trouble. Skip window ledges, radiators, and anywhere hits of sunlight find a way in. I always return bottles to an internal cabinet—top shelf or bottom, doesn’t matter as long as heat and moisture stay out.
Airtight containers help a lot. I’ve lost count of the times a plastic lid warped or someone forgot to twist it all the way shut, only for sticky residue or brown spots to show up later. If you want to stretch the lifespan, glass bottles with Teflon-lined caps work better than clunky plastic. For labs that run hot in summer, a desiccator keeps drama at bay, using drying agents to pull moisture away before it’s a problem.
Labeling and Safety—Lessons Learned
Nothing slows a workday like missing or faded labels. Permanent marker smudges and paper ones peel. I switched to sticky plastic tags and made a rule: no bottle goes back on the shelf unless it claims a clean tag with compound name, date, and my initials. Accidents like mystery bottles littering storage bins become rare when everyone does the same. I’ve watched teams clean up after spills that never needed to happen—all because someone skipped a few seconds labeling.
Better Habits Make for Fewer Nightmares
Nobody needs a fire truck at the lab door. Check bottles every month—crusty buildup or cloudiness tells you to toss the contents. Pair that with good training. Every fresh hire at work spends time learning not just why but how to store stuff right. The lesson sticks far longer than a stack of safety posters.
Smart storage isn’t fancy, and it doesn’t require high drama. A bit of attention and respect goes a long way, whether handling rare compounds or routine basics. My early mistakes proved: good practice starts outside the textbook—and it pays back every single day.
Why Solubility Matters for This Compound
Chemistry labs and industries pay close attention to a compound’s solubility. If you can’t get a substance to dissolve in the medium you need, you start running into real obstacles. N-Methylimidazolium dihydrogen phosphate, bringing together an organic cation and an inorganic anion, lands in an interesting spot for both researchers and those scaling up chemical processes.
How the Structure Influences Solubility
The combination of the N-methylimidazolium cation and the dihydrogen phosphate anion creates a salt with very different behavior compared to simple inorganic salts. Ionic liquids like this one usually show high solubility in water. In fact, add N-methylimidazolium dihydrogen phosphate to water and it gives you a solution without fuss. The hydrogen-bonding capability from the phosphate anion and the polar nature of the imidazolium ring give it a strong affinity for water molecules.
Mix it with less-polar solvents like toluene or hexane, and you see the limits. It rarely dissolves, showing that not all liquids treat this salt equally. Solvents like methanol and ethanol handle the compound better than nonpolars, but not as easily as water. Solvent choice controls how you can use the compound in synthesis, separation, or catalysis.
Why Labs and Industry Pay Attention
Not every solvent-friendly compound behaves well across different temperatures or pH. Research has shown that N-methylimidazolium dihydrogen phosphate holds up in water and can work across a range of acidities, but it’s not infinite. Use it outside moderate temperature or pH, and you can see changes in how much dissolves.
In our group, whenever we needed to run reactions at room temperature, we favored water as a solvent for this compound. It saves money and avoids safety headaches tied to flammable organics. At scale, the high water solubility becomes even more valuable. Water allows you to handle large batches for extraction or catalysis, skipping many steps linked to solvent recovery or waste management.
Where People See Challenges
One problem that keeps showing up is salt precipitation after evaporation. Use it in a reaction, and sometimes you wind up fishing crystals out of your product mixture because water leaves and the salt says behind, unwilling to stay dissolved. This can clog filters, slow purification, and waste your product. Solubility changes at higher concentrations or lower temperatures make some researchers feel like they’re on a tightrope—push things too far and you lose your smooth workflow.
Some Straightforward Solutions
Handling solubility issues starts with clear planning. Matching solvent, concentration, and temperature can keep this compound dissolved when you need it. For larger processing, staged dilution and careful evaporation stops the salt from dropping out unexpectedly. Many chemists mix in a co-solvent like ethanol with water when they sense trouble ahead, which can boost solubility while keeping things safe and affordable.
Down the line, improving reactor design—agitation, temperature control, and efficient solvent recovery—take a lot of sting out of crystallization surprises. Routine checks on solubility curves let you alter the plan before problems hit the lab bench. It’s all about working with the compound’s personality, not trying to bully it into a box built for something else.