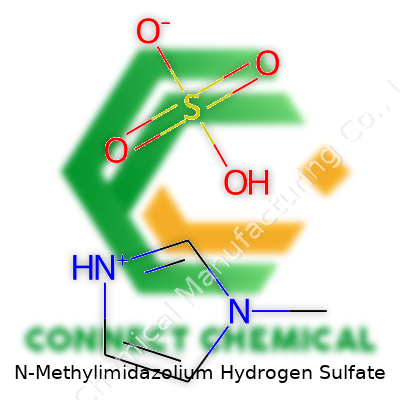
N-Methylimidazolium Hydrogen Sulfate: A Deep Dive Into Its Chemical Journey and Significance
Historical Development
In the late twentieth century, the rise of ionic liquids came on strong, driven by the need for cleaner solvents and better catalysts. N-Methylimidazolium hydrogen sulfate drew attention for its blend of stability and useful reactivity. Its roots tie back to researchers who sought alternatives to volatile organic solvents and stumbled upon the imidazolium backbone as a promising structure. This compound grew in laboratory circles, gaining a reputation for resilience and versatility, especially in fields chasing green chemistry. Over several decades, researchers built a clearer picture of its reactions and practical value, moving from small-scale curiosity to wide-scale applications across synthetic chemistry and industrial processes.
Product Overview
N-Methylimidazolium hydrogen sulfate falls under the family of ionic liquids—salts that show liquid behavior at low temperatures, unlike table salt or rock salt. Designed with a methyl group at the nitrogen position, this molecule links an imidazolium cation with a hydrogen sulfate anion. Its appearance brings a colorless to light yellow liquid. Those who have worked hands-on with this substance know it’s heavier and more viscous than water, making it a distinct choice for blending with other chemicals or using as a reaction medium.
Physical & Chemical Properties
This compound resists easy evaporation, sidestepping the risks tied to solvent fumes. Right around room temperature, it holds steady as a liquid, usually ranging in melting points from below 0°C to slightly above, depending on purity and handling. Heating reveals impressive thermal stability, with decomposition temperatures often past 200°C. It shows strong ionic conductivity—an important feature for many modern applications in electrochemistry. Water dissolves this salt quickly, but it stands strong in acidic environments and doesn't easily form troublesome side products. The viscosity may pose a hurdle for high-speed mixing, though in the right setups it provides control and consistency where it counts.
Technical Specifications & Labeling
Suppliers provide this chemical with purity levels topping 97%, striving for low water content to keep reactions predictable. Labels mark its CAS number (46260-28-4) and identify storage needs: keep the container closed, shielded from moisture, and avoid open flames. Most bottles carry hazard pictograms and caution statements about eye, skin, and respiratory irritation. Molecular weight clocks in at 180.20 g/mol. The liquid sports densities often just above 1.2 g/cm³, and batch certificates include detailed testing reports for residual impurities. Documentation supports safe lab work and process scaling.
Preparation Method
To make N-methylimidazolium hydrogen sulfate, labs combine N-methylimidazole with sulfuric acid under controlled cooling and stirring. This reaction steps through proton transfer, with the imidazole capturing a proton from sulfuric acid. Direct mixing sets off noticeable heat, so glassware cooling and gradual acid addition matter. Solvent-less routes cut down on waste and keep the synthesis process more sustainable, a goal that fits modern priorities. Once reaction finishes, washing and drying yield a clear product or thick oil, depending on the isolation method.
Chemical Reactions & Modifications
Chemists value this compound both as a catalyst and as a solvent, especially in reactions that call for acidity without harsh mineral acids. Used in organic synthesis, it helps create esters, ethers, and other products where selective proton donation makes a difference. Pairing with metal salts or organic bases pushes the scope even further, fine-tuning reactivity by swapping anions or functionalizing the imidazolium core. Some teams explore structural tweaks—adding longer alkyl chains, introducing aromatic groups, or blending with other ionic liquids—to adjust melting point, solubility, or acidity.
Synonyms & Product Names
Chemical lists and supplier catalogs mention names such as 1-methylimidazolium hydrogen sulfate, N-methyl-1H-imidazol-3-ium hydrogen sulfate, and methylimidazolium bisulfate. Some brands label it as an ionic liquid catalyst for ease of recognition while others use system names that sequence the atoms present. These synonyms help users cross-check documentation and avoid mix-ups with similar salts or compounds with minor substitutions.
Safety & Operational Standards
Every handler should treat N-methylimidazolium hydrogen sulfate with respect. Lab gloves, goggles, and good ventilation guard against accidental splashes or vapor inhalation. Spillage brings its own risks, as the compound can cause skin and eye irritation on contact. Chronic exposure, even at low doses, remains poorly studied, so minimizing accidental skin contact stands as best practice. Disposal follows strict regulations since ionic liquids may not break down easily and sometimes harm aquatic environments. Some research teams note its low volatility as a positive, reducing the risk of inhalation hazards compared to classic acids or solvents. Still, open bottles should never be left unattended on the bench.
Application Area
In practice, N-methylimidazolium hydrogen sulfate fills a set of key roles. Organic synthesis laboratories reach for it as both a solvent and acid catalyst—streamlining processes and cutting down on waste from traditional mineral acids. Electrochemical researchers push its use in batteries and capacitors, citing high ionic conductivity and stability as strong points. Some teams in extraction chemistry show that it efficiently pulls metals or organic compounds out of complex mixtures. Research into cellulose processing finds the compound helps unlock renewable materials in greener setups compared to toxic legacy solvents. It’s not limited to the lab, either: some pilot plants and specialty chemical makers bring it into larger reactors, chasing cleaner production streams with less environmental impact.
Research & Development
Innovation rolls forward with this compound at the core of many projects. Scientists target structural tuning—adding groups to the imidazolium ring, shifting the balance of acidity and solubility. New applications spring from high-throughput screening, sifting through libraries of ionic liquids for one that checks the boxes for selectivity, efficiency, and stability. Publications point to advances in asymmetric catalysis, polymer processing, and fuel-cell technology. R&D groups invest in lowering cost of production as the field widens, chasing consistent scale-up from grams to tons. Academic and industrial partnerships build up data on environmental persistence, toxicity, and recyclability, helping guide next-generation design.
Toxicity Research
The research community keeps a sharp eye on long-term safety, testing effects on living cells, aquatic life, and ecosystems. Early toxicity screens show low acute toxicity, at least compared to classic mineral acids or organic solvents. Still, some findings warn about chronic effects in aquatic systems—ionic liquids don’t always break down in wastewater, and trace contamination can stack up in sensitive environments. Studies track breakdown products under sunlight and microbial action, and teams look for new modifications that keep performance high while lowering potential for bioaccumulation or DNA damage. Questions about endocrine disruption and chronic toxicity fuel further research, all pushing towards a stable and responsible safety base before scaling up production and use.
Future Prospects
Looking ahead, N-methylimidazolium hydrogen sulfate has room to grow across multiple industries. Pushes for cleaner manufacturing, less hazardous solvents, and more energy-efficient chemical processes should drive broader adoption. New research into greener chemistry pushes the boundaries on recycling and reuse, as process engineers and synthetic chemists alike press for less waste. If ongoing studies into environmental impacts can guide safer formulations and better treatment technologies, this compound can help cut pollution from large-scale chemical plants to academic labs. As the price drops and data confidence grows, its use could touch everything from pharmaceuticals to specialty polymers, and even renewable energy devices.
Digging into Its Core Functions
Not every chemical grabs headlines, but N-Methylimidazolium hydrogen sulfate deserves a bigger stage in the discussion about making industry cleaner and smarter. I’ve spent years seeing how labs and factories scramble to find alternatives to harsh solvents or outdated catalysts. This ionic liquid often emerges as a reliable team player. Its main job? Creating a safer, more efficient path for chemical reactions, especially those that used to need dangerous or messy conditions.
Chemists turn to N-Methylimidazolium hydrogen sulfate when they need a strong acid without the unpredictability of traditional sulfuric acid. The molecule goes to work in organic synthesis, helping cobble together pharmaceuticals, dyes, and perfumes. I've watched it simplify tricky esterification and alkylation reactions. Chemists like its thermal stability and low volatility. There’s less smell, less evaporation, and less worrying about ruined production batches if the temperature drifts.
Supporting a Cleaner Future
Green chemistry usually comes down to one word: replacement. Industry looks for swaps—cleaner for dirtier, safe for risky, efficient for wasteful. N-Methylimidazolium hydrogen sulfate ticks those boxes. The thing about ionic liquids is that they rarely hit the air as vapor or leak into groundwater, so the risk for lab workers and the environment drops. The lack of contamination is a relief after years of dealing with flammable or toxic solvents that call for special disposal.
There’s a ripple effect in manufacturing. The pharmaceutical sector, for example, jumped at the chance to cut down on hazardous waste. My own experience with lab cleanups makes me appreciate any chemical that trims an afternoon of hazardous solvent disposal down to a five-minute routine wash-up. Manufacturers also see higher yields with less fuss. That means lower production costs, less pressure on raw material supply, and easier scale-ups—benefits felt from the chemist’s bench to the warehouse.
Challenges and the Push Toward Solutions
No chemical solves everything. Labs sometimes struggle with purifying products after using ionic liquids, since these materials cling to whatever they touch. Recovery and recyclability present real headaches. From countless meetings with engineers, I learned that closed-loop systems—where the liquid can be filtered and reused—are being improved year after year. Experts push for innovations in membrane technology, hoping to streamline these steps.
Worries about toxicity don’t disappear just because a solvent stays out of the air. Researchers keep a close watch on how ionic liquids break down. The safety profiles of substances like N-Methylimidazolium hydrogen sulfate get tested regularly, especially as they head toward the wastewater stream. Regulatory agencies want proof that swapping old nasties for new ones doesn’t just kick the can down the road.
Looking ahead, education matters as much as the chemistry itself. Chemists need to learn how to handle these alternatives safely, and plant managers need to invest in staff training. There’s no shortcut here. The urge to shift toward green chemistry means putting safer, smarter procedures in everyone’s hands.
The Chemical Formula and Its Foundation
N-Methylimidazolium hydrogen sulfate holds the formula C4H7N2+ · HSO4–. With a cation formed by methylating imidazole on the nitrogen atom and an anion coming from hydrogen sulfate, this compound brings together two familiar pieces to build something unique and useful. Chemists often write its formula more compactly as [Hmim][HSO4], where Hmim stands for the N-methylimidazolium ion. I remember learning how this small tweak—sticking a methyl group onto the nitrogen—completely changes the molecule’s properties. Suddenly you get a liquid at room temperature, with the ability to dissolve a whole range of other chemicals.
Why This Chemical Matters
My introduction to ionic liquids, like N-methylimidazolium hydrogen sulfate, came during a research project focused on reducing waste in organic synthesis. Traditional solvents make up a huge chunk of chemical industry pollution. In contrast, ionic liquids often give chemists the chance to run reactions at lower temperatures and with less toxic byproducts. This particular compound outshines many conventional solvents because of its thermal stability and very low vapor pressure. Fume hoods still play their role, but the air stays a lot cleaner without volatile organic compounds floating around.
In academic literature, these “greener” ionic liquids show up again and again as solvents, catalysts, and even electrolytes for batteries. I’ve seen colleagues in the lab grab a small vial of [Hmim][HSO4] and turn what used to be a smelly, multi-step process into a one-flask operation. Hydrogen sulfate brings acidity into the mix, making it valuable for tasks such as esterification, extraction, and other acid-catalyzed reactions.
The Challenge of Widespread Adoption
Of course, nothing about chemical manufacturing comes without trade-offs. Ionic liquids don’t evaporate easily, but they can still slip into the water when handled carelessly. Large-scale production means paying attention to their biodegradability and toxicity in real-world scenarios. Over the years, I’ve learned that “green” labels in chemistry only mean as much as the follow-up data on environmental impact, including what happens after industrial use.
Cost and availability give pause, too. Many ionic liquids, including N-methylimidazolium hydrogen sulfate, are made using specialty steps that drive up price compared to off-the-shelf solvents. Over time, technical advances have nudged prices down and created cleaner synthesis methods. I’ve worked with teams switching from classic strong acids to ionic liquids and watched waste disposal costs drop—because there’s less to incinerate and less risk to workers handling dangerous fumes.
Finding Smarter Ways Forward
Bigger change comes not from any single innovation, but from slow, steady shifts as more chemists pick up ionic liquids like [Hmim][HSO4] and adapt them to new uses. Green chemistry education has become critical; folks taking extra care with waste management, learning to recycle these liquids, and looking closely at toxicology reports before scaling up. Cross-industry collaboration helps too, since chemical manufacturers share their successes and warn others off failed shortcuts.
Labs and companies tackling solvent waste or seeking alternatives to petrochemical inputs should consider integrating N-methylimidazolium hydrogen sulfate into pilot studies. By blending practical experience with up-to-date research on safety and life-cycle analysis, the path grows clearer toward using these chemicals responsibly. Whether shortening reaction times or freeing workers from hazardous fumes, the right formula often makes a bigger difference than high-tech promises or PR buzzwords.
A Closer Look at Laboratory Realities
N-Methylimidazolium hydrogen sulfate has popped up in more and more chemistry labs. People treat it as a neat tool for green chemistry, ionic liquids, or catalyst systems, yet hazards often get less attention. I’ve watched an increasing number of graduate students handle it with only minimal gloves and occasional eye protection. The question about its toxicity doesn’t just belong in dusty technical data sheets. Anyone handling this compound should know what might happen if things go sideways.
What Researchers Have Reported
This substance isn’t a household name, so calls to poison control about it don’t come flooding in. Small academic studies, though, address the risks. N-Methylimidazolium hydrogen sulfate belongs to a group called ionic liquids, which people once advertised as “green” because they don’t evaporate easily. It’s important not to fall for that marketing line. Just because something has low vapor pressure doesn’t mean it’s gentle. I remember a synthetic organic chemist who got a splash on her skin and described burning redness and irritation that lingered for days. Some ionic liquids get into the deep layers of your skin, doing more harm than most expect.
Manufacturers warn about strong corrosivity, and the MSDS lists risks for severe eye damage and burns, along with respiratory irritation from dust or aerosols. I checked a few toxicology studies. Some imidazolium-based salts mess with aquatic life at low concentrations, harming the growth of algae and invertebrates. Scientists flagged them as emerging water pollutants, which means the worries aren’t only about direct human contact—ecosystems matter too.
What Makes This Salt Risky
Chemistry tells a story about structure leading to hazards. The hydrogen sulfate part gives the compound a sharp acidity, enough to hurt living cells. N-methylimidazolium mixes that acidity with the ability to push itself through biological membranes, including skin. Once inside, these salts don’t break down right away. Human enzymes don’t quickly chop up ionic liquids, so they linger and their full impact still isn’t mapped out. Chronic exposure remains a big unknown. In my experience, few labs have proper disposal steps, so pours down the drain still happen.
No reliable evidence says this compound causes cancer, but just lacking long-term data doesn’t deliver a green light for careless handling. Several European agencies treat ionic liquids with caution, suggesting gloves, goggles, and chemical fume hoods, and limiting storage with strong oxidizers or bases. It’s simple: treat with respect.
Smarter and Safer Lab Practices
Lab culture shapes risk just as much as the chemical bottle on the shelf. I’ve tried to nudge students toward better behaviors—labeling all containers clearly, swapping out old, degraded gloves, and cleaning up spills fast, no questions asked. Real safety culture shows up when people support each other in calling out shortcuts. For folks using N-Methylimidazolium hydrogen sulfate, I’d push for closed handling systems, better engineering controls, and stricter waste protocols. Local waste collection programs often welcome these substances, but few researchers actually connect with them for safe disposal.
Education holds the key. Young chemists shouldn’t trust “green chemistry” labels to mean “safe chemistry.” Together, knowledge, vigilance, and the right tools prevent stories about hasty accidents or new pollutants. As more chemists experiment with ionic liquids, straight talk about toxicity and hazard gives us the best shot at working safely—and leaving less mess for everyone downstream.
Understanding the Material
N-Methylimidazolium hydrogen sulfate doesn’t pop up on most people’s radar, but it lands in labs and industries where folks lean on ionic liquids for clean energy work, catalysis, and specialty chemistry. This particular compound won’t startle anyone with a dangerous reputation, though that never signals carte blanche. Eye and skin irritation, breathing in vapors or dust, and a general sense of keeping materials locked down for safety all show up in real-world chemical handling. It always pays to treat even the less-known chemicals with healthy respect.
Storage Conditions Matter
Moisture and air love to meddle with certain chemicals, and this one isn’t an exception. Leaving N-Methylimidazolium hydrogen sulfate out in the open brings on clumping, unwanted reactions, and the loss of purity. Humid air draws water right into the powder, causing it to cake up or degrade, which can toss experiments or processes off track. Sealed containers play a huge role in keeping the chemical dry. Glass bottles or high-density polyethylene work well, paired with snug-fitting caps. Every chemist I’ve worked alongside relies on this simple but crucial step.
Temperature: Not Just a Number
Room temperature storage suits this material just fine — most research groups keep it in cabinets set at about 20-25 °C. This stops it from melting, reacting, or breaking down. Letting the room heat up or swinging cold runs risks of condensation and slow changes in chemical makeup. Fluctuations in temperature add unnecessary hurdles, so climate control is more than just a comfort feature. Relatively steady indoor conditions reduce cross-contamination and accidents.
Avoid Mixed Company
Mixing incompatible chemicals, even in storage, opens the door to trouble. Acids and bases, strong oxidizers, and reactive organics shouldn’t bunk together. N-Methylimidazolium hydrogen sulfate won’t explode on its own, but it still doesn’t belong near bleach, peroxide, nitric acid, or strong reducing agents. It makes sense to use segregated shelves with clear labeling. One former coworker set up color-coded tape around storage spots — a small tweak but it keeps mistakes from turning into lab emergencies.
Labeling and Traceability
Clear, durable labels make a world of difference in safety and long-term reliability. Every bottle at my last job bore the full chemical name, date received or opened, and all hazard symbols in plain view. If regulatory groups ever knock on the door, they look at this first. Missing labels spell confusion and lost time, and that’s what typically causes people to mishandle resources.
Accessorizing: PPE and Spill Kits
Nobody likes a chemical spill, but planning beats panic anytime. Storing spill kits specifically made for acid clean-up next to storage cabinets saves the day if accidents strike. Gloves, goggles, and lab coats keep skin and eyes out of trouble, turning chemical handling from a gamble into routine work.
Building a Culture of Safety
Every step in storage, from dryness right down to labeling, leaves its mark on safety. Mistakes usually bubble up from cutting corners — not following basic rules, skipping gloves, or mixing up bottles causes most close calls I’ve seen. Rethinking storage isn’t about fear — it’s common sense. Each person has a role in building trust in a lab or warehouse, and it starts right at the storage shelf. Reliable storage for N-Methylimidazolium hydrogen sulfate grows from simple habits: keep it dry, keep it closed, keep it organized, and always keep people informed.
Why Chemists Track Compounds by CAS Number
Every chemical, whether found in a lab or used in large-scale manufacturing, gets a unique identifier called a CAS number. For N-Methylimidazolium Hydrogen Sulfate, the CAS number 262297-13-2 serves as its tag, making it easy to distinguish from other substances. The idea might seem technical, but for anyone who has spent time searching through catalogs or safety data sheets, these numbers simplify a lot. Instead of wading through long chemical names that look and sound similar, you punch in the CAS and get the right substance, avoiding nasty mix-ups.
Beyond the Number: Why N-Methylimidazolium Hydrogen Sulfate Matters
This compound pops up in several areas of chemistry. Its uses stretch across making ionic liquids, supporting green chemistry initiatives, and even finding roles in organic synthesis. As research shifts more toward sustainability, demand for chemicals offering less environmental harm continues to rise. The imidazolium-based ionic liquids, including those with the hydrogen sulfate anion, help in designing cleaner alternatives to harsh solvents. Working with ionic liquids, especially those like N-Methylimidazolium Hydrogen Sulfate, feels less risky because they don’t evaporate or ignite as easily. That’s a relief for anyone who’s ever worried about the hazards in a busy lab.
Safety Depends on Accuracy
Mislabeling a chemical jar or plugging the wrong substance into a reaction throws off experiments. Even worse, mistakes with identification might lead researchers or workers to overlook proper storage, handling, or disposal rules. Back in my earliest days in the lab, I once mistook two similar-looking imidazolium salts due to unclear labeling. Since then, those CAS numbers have become a regular part of how I double-check everything, especially when ordering new materials. Mistakes get expensive or dangerous fast, and nobody wants to clean up after a spill from rushing through a chemical order form.
The Role of Regulation and Trust
Regulators, suppliers, and researchers speak a common language through these unique numbers. Chemical suppliers use the CAS number 262297-13-2 to show customers exactly what they’re buying, and global safety agencies rely on them for tracking toxicology or environmental impact data. According to guidelines from organizations like the European Chemicals Agency, the accuracy of these details props up everything from workplace safety to environmental protection. Mismatches or errors slow down research, force recalls, or in the worst cases, trigger regulatory headaches that spill into the courtroom.
Supporting Solutions That Build Trust
Getting information right at the source remains the best approach. Manufacturers need to keep their digital and printed material updated, making sure no outdated names or numbers confuse suppliers or customers. Labs should train new staff early on about using CAS numbers for ordering and for logging materials. Digital databases connected to barcodes and handheld scanners speed up this work and trim down mistakes.
With digital tools more accessible than ever, improving traceability across the supply chain pays off for everyone. Less time spent fixing mix-ups means more time for doing real research. Plus, sharing knowledge about these numbers with students and industry newcomers safeguards chemical safety standards into the future. In a world that relies on careful detail, the humble CAS number does a whole lot of heavy lifting, especially for compounds like N-Methylimidazolium Hydrogen Sulfate.