N-Methylimidazolium Tosylate: A Deep Dive into Its Journey, Properties, and Future
Historical Development
Long before the buzz around ionic liquids filled conference rooms and journal columns, researchers were sifting through hundreds of imidazole derivatives hoping to redefine solvent systems. The story of N-Methylimidazolium Tosylate stretches back to those early days in the late 20th century, during a time when many scientists dismissed ionic liquids as academic curiosities. The foundational work started when curiosity about the basicity of the imidazole ring meshed with interest around weakly-coordinating anions. Labs in Europe and Asia, especially those focused on green chemistry and organocatalysis, helped set the stage for practical use. This compound’s emergence as a major ionic liquid wasn’t just luck; it relied on deliberate research investments, insights from electrochemistry, and the push for safer, non-volatile solvents. The chemical industry gradually recognized the value as new regulatory pressures forced traditional solvents into retirement. Today, practically every catalog from a research chemical supplier lists N-Methylimidazolium Tosylate, evidence of a long journey from early trials to established status.
Product Overview
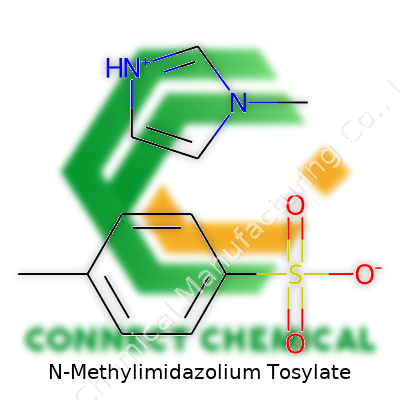
Think of N-Methylimidazolium Tosylate as a salt whose cation combines pi-rich aromatic stability with just enough flexibility thanks to a methyl group. The bulky tosylate anion adds weight and influences solubility and thermal stability. Bottles shipped from suppliers arrive either as viscous clear liquids or low-melting-point solids, sealed tightly for reasons tied to hygroscopicity. The structure, with its methyl-imidazole base and para-toluenesulfonate, leads to broad chemical compatibility and often distinguishes it among ionic liquids for its reliability in catalysis and electrochemical settings. The catalog numbers differ widely, but research chemists usually recognize the characteristic labeling and safe-packaging standards.
Physical & Chemical Properties
This compound’s melting point hovers just below room temperature, often sitting around 20–30°C under dry air. It dissolves in polar solvents with ease and demonstrates excellent thermal stability. A unique attribute lies in its low vapor pressure, making it unlikely to form airborne residues during use—a huge plus where workplace safety comes under scrutiny. Density falls just above that of water, and electrical conductivity supports work in advanced battery research. The combination of chemical inertness toward many common organic reagents and the ability to dissolve a surprising range of organic and inorganic material makes this ionic liquid a favorite for both bench scientists and process engineers. I’ve personally watched it outperform traditional solvents during metal-catalyzed couplings.
Technical Specifications & Labeling
Chemical suppliers list N-Methylimidazolium Tosylate by purity, often 98% or greater, and provide detailed chromatographic and spectroscopic data. Barcoded safety labels state batch numbers and handling warnings—look out for standard hazard symbols signifying irritancy and environmental risks. Every reputable vendor includes GC, NMR, and MS spectra PDFs for lot traceability. Material Safety Data Sheets alert users to the absence of major flammable hazards, but recommend gloves and goggles due to the compounding effect of methylimidazole-based irritation. Regulatory documentation echoes current REACH and GHS standards.
Preparation Method
Laboratory synthesis begins with methylation of imidazole, using methyl iodide or dimethyl sulfate under controlled conditions, followed by anion exchange with sodium tosylate. Crystallization and drying steps demand patience; residual solvents seriously impact purity, which research has shown to alter reaction yields and selectivity. Many labs have replaced single-use glassware with PTFE-lined vessels to cut down on decomposition and waste. The selection of solvents and water scavengers influences final product consistency and shelf stability. Research teams, including mine, have produced gram-to-kilo batches for collaborative projects after optimizing the water-ethanol blend and post-synthesis filtration procedure.
Chemical Reactions & Modifications
One aspect appealing to synthetic chemists is this ionic liquid’s versatility as a reaction medium. It boosts yields in Suzuki and Heck couplings, even under ultra-low catalyst loadings. The tosylate anion brings weak nucleophilicity, ensuring it doesn’t interfere with transition metal complexes or sensitive organometallic intermediates. Chemists tweak the imidazolium core to attach different alkyl groups or introduce chiral auxiliaries, taking advantage of the solid base offered by the tosylate salt. I’ve found the methylimidazolium cation works well in facilitating late-stage functionalizations, particularly where base-sensitive substrates show poor tolerance to traditional conditions. Post-synthetic purification sometimes involves column chromatography, since side reactions can slot in aromatic impurities. With further derivatization, researchers harness this platform to make task-specific ionic liquids designed for CO2 capture or biotransformation.
Synonyms & Product Names
N-Methylimidazolium Tosylate goes by a swirl of synonyms: 1-Methylimidazolium p-Toluenesulfonate, [Hmim][Ts], and methylimidazolium tosylate. Commercial labels reflect subtle variations—some stick with [MIM][OTs], while others write out the structure. Always cross-check registry numbers and synonyms to avoid confusion, especially since regional suppliers use slightly different trade names. Literature provides ample coverage under all forms, so a basic search will surface application notes and safety data under many aliases.
Safety & Operational Standards
On the bench, safety shouldn’t just mean minimizing spills; it’s about long-haul protection from hidden risks. Although lower vapor pressure limits inhalation, exposure to skin or eyes causes serious irritation. Engineering instructors teach new graduate students to work in fume hoods—gloves, lab coat, chemical-resistant goggles always on. Spill trays and secondary containment containers mitigate drops and leaks. Waste streams containing N-Methylimidazolium Tosylate must go to specialized disposal, not down the drain, because regulatory bodies have flagged persistent toxicity concerns in aquatic environments. Regular stock inspections curb degradation from humidity pickup. My habit has been to copy every lot’s MSDS summary to a visible spot on the lab wall, making sure mistakes get caught before they end in phone calls to EH&S.
Application Area
From bench-top organic synthesis to full-scale industrial catalysis, application breadth keeps expanding. Batch and flow chemistry groups rely on it for biocatalysis, CO2 fixation, and electrosynthesis. Electrochemical devices, including next-generation supercapacitors and rechargeable batteries, look to ionic liquids like this one for higher ionic strength and reduced volatility—key reasons why battery startups order this compound in bulk. The pharmaceutical sector uses it to stabilize reactive intermediates and push green chemistry agendas forward, chasing solvent-free or recyclable system mandates. Labs at several research universities push boundaries through metal extraction and separation processes using this salt, pointing to the compound’s non-flammability as a selling point for plant safety managers. I’ve seen colleagues win grants by tying application notes into the sustainable-development appeal.
Research & Development
The research base around N-Methylimidazolium Tosylate reflects the rapid pace of ionic liquid innovation. Every year, dozens of new publications surface exploring task-specific uses, novel derivatives, and greener synthesis strategies. Scientists work to shrink the environmental footprint through improved waste management protocols, biocompatibility testing, and lifecycle analysis. Grant-supported collaborations between university labs and chemical manufacturers push digital modeling for process scale-up and computational screening for new catalyst pairings. Workflows for continuous-flow processes, real-time product monitoring, and device fabrications rely on robust, reproducible batches. The endless search for better performance in electrochemical and separation science keeps this compound on the radar for students and senior researchers alike.
Toxicity Research
Studies show low acute toxicity for mammalian cell lines, but environmental persistence and chronic impact concern regulatory agencies. Bioaccumulation in freshwater organisms leads to regulatory interest and drives ecotoxicology studies in universities across Europe and East Asia. Some evidence points to inhibition of microflora in soil and water—factors that have led to guidelines limiting industrial release. Chronic exposure and dermal contact studies inform workplace exposure limits recommended by international agencies. In my own work, strict adherence to containment and disposal standards help keep exposure below these regulatory limits. The need for more long-term multi-generational studies and aquifer safety assessments stands clear from speaking with colleagues at environmental institutes.
Future Prospects
Looking forward, research points toward the next generation of N-Methylimidazolium Tosylate derivatives: chiral, fluorinated, or task-specific versions, each with tailored reactivity and minimized environmental footprint. The push for circular economy principles in the chemical industry drives interests in recyclability and degradability. Major companies look for process-friendly ways to regenerate and re-use ionic liquid stocks, cutting down on both cost and toxic waste. Developments in bio-based feedstocks for tosylate anion precursors may allow manufacturers to shrink carbon footprints further. Integrating predictive toxicology software into product design lets development teams identify harmful pathways before products enter the market. The potential to merge new safety data, digital chemistry, and greener manufacturing may transform N-Methylimidazolium Tosylate from an industry workhorse to a vanguard of sustainable chemistry.
Breaking Down the Basics: What Is N-Methylimidazolium Tosylate?
N-Methylimidazolium Tosylate stands out in the world of chemistry labs. It looks like a small white powder on a shelf, but its role in life sciences and green chemistry is much bigger than its appearance. Every so often, a chemical like this draws more attention because of the work it quietly gets done behind the scenes.
Key Roles in Chemical Reactions
N-Methylimidazolium Tosylate earns its keep as an ionic liquid. Chemists like to call it “task-specific.” Unlike those harsh, volatile solvents from years past, this compound mellows out most reactions. Labs use it for catalysis—basically to help reactions finish quicker or go under gentler conditions. For instance, it gets used in forming carbon-carbon bonds, something every organic chemist cares about. Making pharmaceuticals hinges on linking molecules in the right way. N-Methylimidazolium Tosylate helps there, making these reactions smoother and cleaner. Less toxic waste, more product. That’s always welcome—nobody enjoys wrestling with hazardous byproducts late at night.
Green Chemistry’s Helping Hand
Green chemistry doesn’t just mean swapping out chemicals for ones that sound less scary. It’s about changing how reactions happen in the first place. For years, labs struggled to move away from those old, polluting solvents like benzene or chlorinated compounds. N-Methylimidazolium Tosylate offers a different route due to low volatility. No sharp, stinging smell in the air means fewer headaches—literally and figuratively. The compound doesn't evaporate easily, so emissions drop, and there’s less pressure to keep reactions airtight or to filter exhaust constantly.
Solubilizing and Extraction Power
N-Methylimidazolium Tosylate finds its way into material science and biochemistry, often as a solvent that can hold onto both polar and nonpolar compounds. For those who’ve ever had to pull natural products out of crude plant extracts, that versatility means fewer steps to chase your target molecules. In real-world applications, that equates to better yields and less time cleaning up unwanted byproducts. This comes into play with biomass conversion too—turning agricultural waste into useful chemicals without using the harsh stuff from decades ago.
A Push for Safer, Cleaner Industry
The chemical industry faces heat over safety. Stories of accidental spills and toxic clouds have left their mark. The push for alternatives means a steady look at options like N-Methylimidazolium Tosylate. The facts back it up: lower flammability, less corrosive action on gear, and safer disposal compared to a lineup of traditional solvents. Universities and startups both keep bottles in their cabinets for a reason.
Challenges on the Path Forward
There’s no magic bullet in chemistry. Ionic liquids, including this one, sometimes come with problems if they build up in water streams or react in ways nobody saw coming. Careful review and monitoring help keep things on track. Industry leaders could invest more in recycling these liquids and developing closed-loop processes, so what goes in can often get cleaned and reused. Compared to the solvents that kicked up trouble in the past, this feels like a safer bet that just needs tighter controls and better data over time.
Breaking Down the Chemistry
Chemists often reach for N-Methylimidazolium Tosylate when they want a reliable ionic liquid for organic synthesis or electrochemistry. At its core, the structure brings together two main players: the N-Methylimidazolium cation and the Tosylate anion. The cation side, derived from imidazole, holds a five-membered ring packed with two nitrogens and a methyl group attached to one of them. That swap of a hydrogen for a methyl seems minor from the outside, but it tweaks electron distribution throughout the ring. The result changes the way the molecule interacts with solvents and other ions.
The other partner, Tosylate, carries a benzene ring linked to a sulfonate group and a methyl tag on the connection point. This sulfonate isn’t just a passive spectator: it shifts the salt’s solubility, melting profile, and overall stability in solution. I remember struggling with a reaction that would clog up at room temperature. Swapping in N-Methylimidazolium Tosylate kept the mixture smooth, showing how even small chemical differences bring big shifts in lab behavior.
The Role in Modern Labs
The chemical arrangement creates an ionic liquid — a salt that stays liquid at room temperature. These aren’t just curiosities. Ionic liquids like this one have made life easier for researchers and industries chasing cleaner processes or better extraction methods. Their low volatility means technicians don’t have to worry as much about fumes; spill a bit of this stuff, and you pretty much need a mop, not a respirator. That matters in teaching labs where safety trumps speed.
Researchers lean into N-Methylimidazolium Tosylate for tasks like catalyzing sensitive reactions or helping separate rare earths from mixed solutions. IBM showed interest a decade ago, using similar salts in battery research for stability at higher voltages. The structure’s appetite for electrons and ions, shaped by both cation and anion, underpins those results. Practical chemistry rarely comes from abstract theory alone — the proof shows up in the yields and in the ease of cleanup.
Challenges and Future Directions
Trouble crops up, though. Ionic liquids don’t all play well with water, and not all of them break down safely in the environment. Some stick around or break into byproducts that haven’t been fully studied. The methylimidazolium family sometimes resists biodegradation. People sometimes overlook waste handling, but proper disposal of these liquids calls for serious attention. A chemist ignoring waste protocols courts trouble for the next generation.
Finding or building better salts means considering both the molecule’s performance and its end-of-life profile. Teams at green chemistry institutes have pushed for bio-based ionic liquids or for tweaks to the structure, hoping for versions that wash away cleanly or support a circular economy. I once saw a group in Germany try adding extra functional groups to the imidazolium ring. Results were promising but purity and yield remained trade-offs they had to fight through at scale.
From Bench to Industry
N-Methylimidazolium Tosylate represents the current frontier where creative organic chemistry meets real-world challenges. The cation and anion work together to deliver a chemical tool with unique properties, not just a repackaged solvent. Ongoing work to improve these structures, anticipate risks, and plan for safer disposal taps into the sense of responsibility many scientists feel — not just for today’s experiments but for outcomes several steps down the line. Progress in this area rides on details: the kinds of atoms in the ring, the attachments at the edge, the patience to try again.
Getting Real About Chemical Solubility
Anybody who’s spent enough time in a chemistry lab knows the question of solubility has a way of looking simple while hiding bigger implications. Talking about N-Methylimidazolium Tosylate (NMImOTs), that mouthful of an ionic compound, brings us to a crossroads where academic curiosity meets practical application. This isn’t some abstract chemical on a dusty shelf—it’s found work in fields from green chemistry to electrochemistry.
Understanding NMImOTs
This compound draws attention thanks to its ionic liquid nature. Ionic liquids often grab headlines as alternatives to volatile organic solvents. They’re supposed to be safer and easier on the environment. N-Methylimidazolium Tosylate combines a methylimidazolium cation, popular for its stability, with a tosylate anion, recognizable for its bulky aromatic structure.
Diving Into Solubility
Anyone testing NMImOTs in water will find it dissolves—and it doesn’t take much effort. In fact, water readily takes up this salt-like compound, which means researchers and product developers can count on strong mixing. Science backs this up: peer-reviewed journals, such as the Journal of Physical Chemistry B, and supplier data sheets point out how many imidazolium salts, including tosylates, fall squarely into the "water-soluble" category. This follows from the distinct ionic nature of both the compound and the solvent.
Solubility shapes how NMImOTs gets used. Water as a solvent opens doors for sustainable laboratory processes. Someone running a reaction or an extraction often wants to switch away from acetone or ether, and NMImOTs answers by mixing well in water. You see this pop up in published synthesis protocols or when I see other bench chemists designing catalyst platforms. Environmental scientists perk up because water solubility relates to questions of bioaccumulation and waste management—soluble means less risk of persistent residue but also asks for more care in treatment after use.
Why It Matters
Chemists, materials scientists, and engineers care about water solubility. Soluble ionic liquids, like NMImOTs, make processing easier. Recyclability improves since water enables separation steps. For instance, ionic liquids help capture carbon dioxide or remove contaminants when paired with water phases. Personal experience tells me this property often saves headaches by slashing hazardous waste and making cleanup straightforward.
But real-world labs face the flip side—water-miscible chemicals sometimes create harder downstream challenges. Separation or removal from water streams depends on robust protocols. Without good extraction or filtration, researchers risk sending active byproducts down the drain. I remember more than one lab scramble in graduate school, wrangling with separation columns, after a test batch left unexpected residues.
Pushing for Smarter Solutions
Effective lab practice means thinking about the whole lifecycle, not just the start. Water-soluble compounds demand attention not only to yield but also to byproduct fate. That’s where collaborative innovation pays off. Teams now design greener routes for reusing or breaking down N-Methylimidazolium salts after use. Suppliers have even begun offering closed-loop systems, allowing for resin-based recovery or low-energy distillation steps to recycle these liquids directly from aqueous solution.
Solubility isn’t just a number out of a data book. For N-Methylimidazolium Tosylate, the ability to blend so completely with water can either simplify or complicate a project. The key sits in building smart, responsible lab practices, so chemistry supports both progress and stewardship. As labs scale up the use of these compounds, every detail counts.
Practical Experience with Chemical Storage
Storing chemicals like N-Methylimidazolium Tosylate takes more than following a simple guide. This compound, known for playing a role in organic synthesis and catalysis, brings its own quirks to the table. In my years working alongside researchers, I’ve seen overlooked details turn safe labs into risky workplaces overnight. A bit of sunlight, unchecked humidity, or careless placement can shorten the life of even the most stable chemical.
Temperature Matters
Most folks in the lab agree—room temperature isn’t just a comfort zone for people. This ionic liquid stays most stable in cool, dry spaces, typically 15–25°C. Keep it out of direct sunlight, since heat and UV rays can prompt chemical changes that nobody wants. I’ve watched colleagues place glass bottles by windows, thinking the container itself gives enough safety. Give this compound a shady, temperature-controlled shelf to avoid headaches down the line.
Moisture and Air Exposure
Anyone who’s dealt with sensitive reagents knows the trouble that water brings. Once moisture gets in, purity flies out the window and the chemical’s reactivity slips fast. N-Methylimidazolium Tosylate pulls water right from the air—a real desiccant’s nightmare. Always keep the storage container tightly sealed. Use a desiccator cabinet for open bottles if you’re in a humid area. In my experience, even quick opening and closing during rainy seasons invites invisible guests, changing the reliability of reactions without warning.
Choosing the Right Container
Glass tends to work best, since plastics have a habit of leaching or interacting with ionic liquids over time. A sturdy glass jar with an airtight lid beats flashy packaging. An amber bottle helps block out stray light. It sounds simple but grabbing whatever container’s nearby just isn’t worth the risk, especially for expensive or hard-to-replace batches.
Labeling and Segregation
Label every bottle clearly, right down to the date received, lot number, and hazard class. I’ve run into enough mystery jars—no one enjoys guessing games with white powders. Keep N-Methylimidazolium Tosylate away from incompatible substances. Strong oxidizers, acids, and even some bases don’t play nice. Accidental mixing, even in small spills, can cause real trouble. I always recommend keeping a chemical compatibility chart close to where storage decisions happen. It only takes one mix-up to put everyone on edge for weeks.
Routine Inspections and Waste Management
Fresh stock lasts a long time, but chemicals never give a warning before they degrade. Schedule regular checks—look for clumping, discoloration, or strange smells. Dispose of degraded material promptly and safely, following local regulations. I keep a logbook handy for expiry dates, because relying on memory in a busy lab leads to overlooked risks.
Fact-Based Solutions for Better Storage
It doesn’t take fancy technology to improve storage. Invest in a weather-proof, lockable storage cabinet. Train new lab members to check seals and record every withdrawal. Keep a stash of desiccant packs, and replace them once they appear spent. Pay attention to ventilation in the storage room; stagnant air can amplify small leaks. Clean up spills right away, with emergency kit materials within reach. My teams run cleaner and safer labs using basic habits, not just expensive equipment.
Importance of Good Storage Practice
Safely storing N-Methylimidazolium Tosylate protects both people and data. By taking storage seriously, research gains reliability, budgets stretch further, and everyone sleeps a little better at night knowing the lab is a safer place. Real oversight, not just rules posted on walls, makes all the difference in building a culture of responsibility around chemicals like this one.
Looking Closely at N-Methylimidazolium Tosylate
In labs and research industries, ionic liquids like N-Methylimidazolium Tosylate get a lot of attention due to their unique chemical properties. People often want to know if using these materials comes with health risks. News stories sometimes spark concern, especially about new synthetic chemicals, and it’s worth pausing to check the facts before jumping to conclusions.
Understanding the Safety Concerns
This compound’s structure—an imidazolium cation with a tosylate anion—means it shares some chemical traits with other ionic liquids. Ionic liquids can behave very differently from regular solvents. On one hand, these substances can offer advantages: low volatility means less inhalation risk, and high thermal stability reduces fire hazards. On the other hand, new molecules deserve healthy skepticism because the long-term health effects are rarely clear from the start.
What the Science Tells Us
The main safety studies available for N-Methylimidazolium Tosylate point to some mild irritant properties. My time working with various imidazolium-based salts in academic labs taught me to respect unknown compounds—just because something doesn’t burn your skin or smell toxic doesn’t mean it’s harmless. Data on skin or eye irritation, environmental impact, and acute oral toxicity remain sparse. Some ionic liquids break down into less harmful by-products, but others can linger in the environment or disrupt biological systems, especially aquatic life.
No established international agency lists N-Methylimidazolium Tosylate as a major hazard today, but the lack of labeling isn’t the same as proof of safety. The absence of strict guidelines often says more about neglected testing than actual risk. In a few cases, related compounds in the same chemical family have shown moderate toxicity to algae and fish. It’s fair to say much is still unknown here.
The Role of Good Practices
Nothing beats responsible stewardship. I strongly believe that people working with any synthetic chemicals—including those labeled as “green” or “safe”—should follow the classic safety practices: gloves, good ventilation, and proper storage. The packaging for N-Methylimidazolium Tosylate often comes with a note avoiding inhalation, ingestion, and skin contact. This kind of caution might sound repetitive, but it’s there to remind everyone these things haven’t earned the benefit of the doubt. Lab accidents almost always start from little shortcuts or overconfidence with “low-risk” materials.
Future Proofing: What Should Change?
We need regular, transparent toxicological testing of ionic liquids before crowning them as sustainable solutions or safe for everyday use. Companies and research groups can publish thorough studies—covering long-term effects, environmental toxicity, and biodegradability. Knowing whether accidental spills end up in groundwater or vaporize harmlessly makes a world of difference. Regulatory agencies should step in to keep up with innovation, closing the gap between chemistry’s possibilities and real-world safety knowledge.
This kind of attention to detail isn’t about stoking fear or hindering innovation. It’s about protecting workers, communities, and the planet. Skepticism isn’t negativity—it’s an invitation to do science better. Anyone working with N-Methylimidazolium Tosylate or other lesser-known chemicals can help by demanding stronger data before acceptance, practicing rigorous safety, and being honest about what we still don’t know.