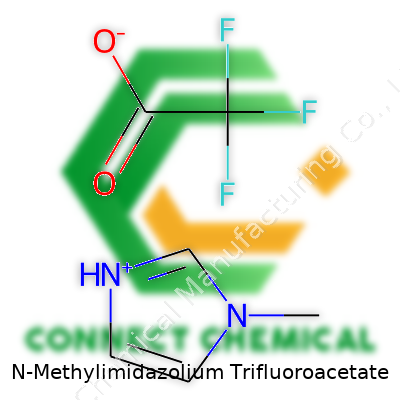
N-Methylimidazolium Trifluoroacetate: A Ground-Level Look
Historical Development
Curiosity and necessity always drive chemists to look for better solvents and ionic liquids. Decades ago, researchers played with imidazole rings and hit on a family that mixed stability with practical handling. Once methylated and paired with different anions, these salts took on new personalities. Trifluoroacetate, with its bold electron withdrawal and low basicity, offered a leap in performance over earlier halide anions, which too often brought corrosion or instability. Labs started swapping clunky, old-school salts for trifluoroacetate-based systems and opened the door for streamlined synthesis, improved catalysis, and cleaner extractions. Manufacturers saw a window for scaling up, responding to academic and industrial demand that kept climbing every year.
Product Overview
The compound N-Methylimidazolium Trifluoroacetate often shows up in liquid or powder form depending on storage, temperature, and what tweaks the producer prefers. It’s not another everyday lab salt. Its blend of an imidazolium body and a highly fluorinated anion makes it a prize for anyone wanting ionic character without a whole lot of fuss. People lean into this material for more than just the charge or stability—its compatibility with a variety of organic and inorganic tasks stands out. In the real world, this product has become a mainstay not only among chemists working on green chemistry or extraction but also for those handling polymerization and catalysis.
Physical & Chemical Properties
It surprises a lot of people just how wide the liquid temperature range can be with N-Methylimidazolium Trifluoroacetate. This salt brings a melting point that sits well below water’s, making it liquid in many standard lab conditions. Odor is faint to none—no harsh smells to fight with—though handling in a fume hood still matters. It carries a density around 1.3-1.5 g/cm³, and stays stable up to 250°C before decomposition raises eyebrows. Water and alcohols break it down slowly, yet hydrocarbons keep their distance. Electrochemical windows are wide enough for battery and catalysis development, letting engineers stretch their imaginations past what traditional solvents give. Conductivity and viscosity don’t just matter for papers; they decide if your process is going to run smooth or clog up halfway through production.
Technical Specifications & Labeling
Both scientists and suppliers must be clear as day about what’s inside the bottle. For N-Methylimidazolium Trifluoroacetate, the standard is high: purity usually hits above 98%. Color matters to most users—anything brown or yellow signals impurities, so top-quality versions stay colorless or faintly yellow. Labels call out CAS numbers, storage advice (“keep sealed, avoid sunlight, dry environment”), and batch numbers for traceability. Packaging often comes in glass or special plastic to stop any reactions with the container itself. If paperwork is missing on safety data or product specs, you might wonder just how trustworthy the source is.
Preparation Method
Making N-Methylimidazolium Trifluoroacetate at scale doesn't just need clean glassware and a calculator. Chemists usually build it from N-methylimidazole and trifluoroacetic acid or one of its activated derivatives. Reactions need tight temperature control since excess heat favors side products. Purification calls for repeated recrystallization or washing with dry solvents, followed by rotary evaporation to recover the pure ionic liquid. Every step pulls on a mix of patience, experience, and chemistry intuition—skip the details and yield or quality will take a hit. Larger batches in industrial settings replace flasks with specialized reactors, but the balancing act between speed and purity never goes away.
Chemical Reactions & Modifications
This salt doesn’t shy away from the action. It steps in as a solvent and sometimes an active participant in alkylation, acylation, and catalytic cycles. The imidazolium structure itself can be functionalized further, introducing ether, alkyl, or aryl groups to tune physical properties, increase solubility, or lock in selectivity during synthesis. Trifluoroacetate’s role isn’t just to support—the electron-poor nature can encourage unusual ion pairing or act as mild nucleophile scavenger. Scientists who crave more out of their ionic liquids will tweak the structure, push the reactivity, and track each outcome with sharp attention.
Synonyms & Product Names
People in labs and factories throw around a lot of names for the same substance. "1-Methylimidazolium trifluoroacetate," "NMI-TFA," or just “methylimidazolium salt” show up on invoices, papers, or safety sheets. The shorthand might confuse newcomers, especially with subtle variations in methyl or positional isomers. Standardized identifiers like CAS numbers and InChI keys help pin down exactly what's in use, keeping everyone straight on what’s in inventory or going into large-scale runs.
Safety & Operational Standards
Nobody wants a surprise when handling specialty chemicals. Even though N-Methylimidazolium Trifluoroacetate brings less volatility than classic solvents, it demands respect. Adequate ventilation isn’t just good practice—it’s a requirement, since trace decomposition may release imidazole or TFA vapor that will irritate airways. Gloves, goggles, and full-length coats feel routine after a while but will save skin and eyesight when accidents happen. Disposal must honor regulations on fluorinated waste and strong bases, a detail some labs miss until regulatory visits come around. Safety data sheets drive home these points, guiding training programs and emergency plans.
Application Area
This ionic liquid has made itself useful well beyond basic organic synthesis. It sits at the center of green extraction experiments, pulling rare earth metals from waste or purifying pharmaceuticals. More than once, battery developers leaned on its wide voltage window to test new electrode formulations. Polymer chemists count on it in ionic polymerizations, using the flavor of the anion and the backbone’s stability to dial in properties that older solvents just can’t deliver. Biotechnologists play with its interactions in enzyme-catalyzed reactions, searching for sharper yields or fewer unwanted byproducts. Its reach keeps growing as curiosity drives scientists into new territory.
Research & Development
Academic labs and R&D teams keep pushing the boundaries with N-Methylimidazolium Trifluoroacetate. Teams working on new energy storage materials, designer solvents, or biocompatible media have run headlong into both the strengths and headaches of this salt. Papers come out every year showing subtle tweaks in structure, reactivity, and application. Grants feed into figuring out how to lower production costs, scale up recycling, and reduce environmental impact. A research group with a few grams will ask different questions than a commercial plant dealing with multi-kilo lots, but across the board, the drive stays focused on higher performance and lower risk.
Toxicity Research
Safety claims don’t come from gut feeling—they grow from well-documented animal and cellular studies. Research has mapped out the acute toxicity of N-Methylimidazolium Trifluoroacetate in rodents and measured cellular impacts in lab dish models. The salt doesn’t leap into the “high hazard” zone, but studies show chronic exposure can upset liver enzymes and cause moderate irritation at high concentrations or long exposures. Environmental scientists keep a close eye on how imidazolium-based ionic liquids break down in natural waters or soils, since fluorinated waste stays persistent longer than many alternatives. Reviews from journals emphasize responsible use and careful tracking, lessons that industry needs to accept if the field expects public trust and continued growth.
Future Prospects
All of the signs point to more interest and wider use of N-Methylimidazolium Trifluoroacetate. As demands for clean energy, selective extractions, and sustainable chemistries climb, researchers expect this compound's portfolio to expand. Cost still needs to drop for large-scale adoption in battery electrolytes or pharmaceutical processing, and the search for green synthesis or recovery methods looms large. Next-generation derivative structures might deliver lower toxicity with the same robust performance. Integration into closed-loop production would limit environmental impact and push adoption beyond high-tech circles. It will take both sharp science and careful stewardship to match the hopes tied to this unique salt.
Everyday Chemistry with Imidazolium Salts
Few people outside chemistry labs recognize N-Methylimidazolium trifluoroacetate by name, but its impact stretches into chemical manufacturing, energy technologies, and environmental work. Standing in a lab, holding a clear vial labeled with this long name, I remembered my first days handling ionic liquids. There has always been something fascinating about salts that turn liquid at room temperature, carrying no tell-tale scent, yet unlocking real breakthroughs in how reactions run.
Why This Salt Matters
Unlike common organic solvents, this compound sits in the family of ionic liquids—salts designed to stay liquid across a big temperature range. N-Methylimidazolium trifluoroacetate holds a special spot among these, providing stability, low volatility, and the ability to dissolve both organic and inorganic stuff. Chemists like it for the flexibility: running complex reactions with less flammable, less toxic liquids makes for safer workplaces. Its low vapor pressure means fewer harmful fumes in the air, supporting safer habits for everyone nearby.
Chemical engineers value this salt for separating materials that otherwise stick together—think breaking tough cellulose apart for biofuel work. I have seen teams spend days frustrated with older solvents, struggling to pull valuable sugars from plant waste. With this trifluoroacetate salt, the task often runs smoother, the yield improved, and the waste produced more easily contained. These improvements never hit the headlines but mean a lot for the people stewarding new energy projects.
Research, Batteries, and Greener Technology
This ionic liquid keeps turning up in battery research. Electrolyte stability matters when designing batteries meant to run for years with minimal risk of leaking or breaking down. Clean, stable conductors stretch battery life and cut down on maintenance. My own favorite example comes from watching students test new prototypes: safer electrolytes mean more learning, fewer setbacks from messy failures. N-Methylimidazolium trifluoroacetate helps teams move past common roadblocks.
Besides batteries, this salt shows up as a catalyst or co-solvent in major research projects. The push towards greener chemistry relies on shifting away from volatile organics, and this salt offers one path. Environmental experts note fewer air emissions and lower toxicity, two changes demanded by both watchdogs and regular folks concerned about industrial impacts. On a personal note, knowing the liquid in your flask stays put rather than filling the room with harmful fumes steadies the nerves during long experiments.
Toward Wider and Safer Adoption
Nothing comes without challenges. Making ionic liquids at scale still costs more than old legacy solvents, and questions linger about how to collect and recycle these chemicals after use. Some labs experiment with recovery systems to filter out spent liquids, readying them for fresh work. Across several projects, I have seen teams saving thousands in replacement costs, simply by investing up front in better recovery gear. While not glamorous, these tweaks shape the chemical industry’s future.
Sharing knowledge about safer, more effective solvents pays off across academic and industrial boundaries. Young chemists, environmental researchers, and manufacturing veterans benefit from understanding how salts like N-Methylimidazolium trifluoroacetate reshape the toolkit. Every safer process, every reduced emission, pushes us closer to an industry that no longer forces a trade-off between efficiency and health. I believe the growth of such chemicals signals real hope for technology and for the everyday people working beside it.
Why Chemical Formulas Matter To Chemists
Every time someone talks about chemical compounds, there’s usually a quick check for the formula. In the lab, no one wants surprises when handling reactive substances. N-Methylimidazolium trifluoroacetate comes up often in green chemistry and ionic liquids research, and people keep searching for its chemical formula: C5H7N2⁺ · C2F3O2⁻. Many scientists and students stumble at this point, either mixing up the structure or missing key elements in the formula.
Breaking Down The Formula
This compound forms from two parts. There’s the N-Methylimidazolium cation, which has a formula of C4H7N2⁺, and the trifluoroacetate anion, with a formula of C2F3O2⁻. Connecting these two pieces builds the full name: N-Methylimidazolium trifluoroacetate, or C4H7N2⁺ · C2F3O2⁻. People sometimes write the combined version as C6H7F3N2O2, but it helps to keep in mind which parts are positive and negative for clear communication in chemical contexts.
Importance in Research
Researchers have been drawn to this compound because ionic liquids open up new possibilities in catalysis, extraction, and electrochemistry. These kinds of salts, like N-Methylimidazolium trifluoroacetate, often replace volatile organic solvents. Many scientists point out their lower vapor pressures and reduced toxicity compared with old-school solvents. The trifluoroacetate anion brings extra stability to the mix, which matters for certain reactions where heat or light would break apart weaker compounds.
Whenever I have worked with ionic liquids in a teaching lab, students always ask for quick and reliable references. Trust in the information grows when data points come from reputable places like peer-reviewed journals or chemical suppliers registered with international regulatory agencies. Merck, Sigma-Aldrich, and other mainstay suppliers publish material safety data sheets that match the formulas presented here, giving extra weight to the accuracy of this information.
Challenges With Naming And Handling
Mix-ups pop up in classrooms and labs when names and formulas don’t match. Some might write “1-methylimidazolium trifluoroacetate” expecting it to be something new, but they’re actually holding the same compound. If a researcher is not careful with notation, failed experiments or mislabeling follow. Having a standard way of writing formulas—using positive and negative breakdowns for ionic compounds—reduces confusion. It also improves safety by helping chemists spot potentially hazardous misidentifications on the shelf.
Trusting Good Data: A Crucial Step
Looking up formulas should never turn into a guessing game. Having reliable resources and cross-referencing with published literature makes a difference. I’ve seen colleagues avoid costly mistakes just by following best practices: checking labels, consulting safety sheets, and sticking to proper chemical formula notation. Transparency from suppliers moves the whole field forward by making sure researchers around the world are talking about the same molecule, not an imposter with a similar name.
Looking Toward Solutions
Clarity in chemical notation and plain access to trustworthy databases play a direct role in lab safety and productivity. If coursework, software tools, and even bottle labels are kept accurate, the next generation of scientists will see fewer preventable mistakes. Researchers agree that keeping up with best standards always beats shortcuts or unclear labels. N-Methylimidazolium trifluoroacetate, for all its scientific intrigue, works best when everyone agrees on the formula: C4H7N2⁺ · C2F3O2⁻.
Getting to Know the Chemical in Question
You don’t hear much about N-Methylimidazolium Trifluoroacetate outside of chemistry labs and tech papers. This ionic liquid holds interest for its ability to dissolve a range of materials and offer conductivity prized in manufacturing and research. Like many innovative chemicals, it’s crept into specialized use before most people even try to say its name. Questions often focus on danger: Could this stuff pose risks to health or the environment?
Looking at Toxicity and Hazards
I’ve spent enough time working with all sorts of chemicals to know one thing—never take a new substance for granted. Long, complicated names sometimes go hand-in-hand with toxicity, sometimes not. Researchers use N-Methylimidazolium Trifluoroacetate for tasks including catalysis or as a solvent in green chemistry approaches. This can give a false sense of security, since “green chemistry” gets thrown around as if it means “totally harmless.”
Toxicity data on N-Methylimidazolium Trifluoroacetate isn’t as public or extensive as what you would find for long-established compounds. Some journals discuss minimal direct effects in short-term lab tests on cells and small organisms. Sounds comforting at first glance, but I’ve seen science change position once long-term exposure gets studied. Ionic liquids as a family sometimes show low volatility, so you won’t breathe much of it in regular rooms. That reduces inhalation risk compared to traditional organic solvents.
The sharper worry comes from ingestion or skin absorption. N-Methylimidazolium Trifluoroacetate includes the trifluoroacetate anion, which can carry toxicity in higher exposure. In animal studies relating to similar compounds, effects on liver function and metabolism have come up after strong exposures. At low doses, effects appear mild for now, but labs rarely verify this for the general working population across years.
Environmental Fate and Persistence
Splashing chemicals down drains never makes sense, no matter what you read on “green” alternatives. N-Methylimidazolium Trifluoroacetate breaks down more slowly in nature compared to traditional solvents. Ionic liquids don’t evaporate quickly, so they stick around in soil and water. Some research shows persistent organic ions build up in aquatic organisms, although definitive evidence specific to this chemical needs more data. The fluorine content, as several scientists point out, should raise an eyebrow given concerns about environmental accumulation (think PFAS debate—perfluoroalkyl substances have caused headaches for regulators worldwide).
Solutions and Smarter Handling
My approach stays cautious, especially since these ionic liquids look promising but lack years of field evidence regarding safety. Workers using this chemical should receive gloves, face protection, and splash-resistant clothing. Well-ventilated spaces cut down on surface contamination and accidental exposure. Safety data sheets remain a must, even if regulatory agencies haven’t finished risk assessments. Waste should always go to chemical disposal—not down the sink—because community water health finishes last if we don’t respect the unknowns.
Decades in chemistry teach me not to fear every new ingredient, but to ask hard questions nobody answered yet. Would I keep N-Methylimidazolium Trifluoroacetate out of casual use? Yes, until we know about buildup in people and the environment across real time. Responsible research and transparency matter just as much as discovery. New chemicals can solve big problems—but if we ignore basic safety, those solutions fade fast.
Keeping Science Practical: Why Storage Matters So Much
Ask anyone who’s worked in a chemical lab long enough, and their stories will carry a common theme—little details make the biggest difference. One bottle on a high shelf with the wrong cap or broken seal can mean wasted material, risk, or worse. I’ve seen everything from cracked glass to confused labels, and it never ends well. With chemicals like N-Methylimidazolium Trifluoroacetate, carelessness quickly turns into a hazard for people and projects.
Temperature and Humidity: Control Isn’t Optional
This compound reacts with water in the air. If the stopper lets in too much moisture, performance tanks, and contamination builds. A dry atmosphere, ideally below 60% humidity, keeps the salt stable. Refrigeration down to 2–8°C works, but there’s a twist—untrained hands might just toss it next to a lunch bag or a bottle of acid, which creates new risks. Fume hoods help, but a true chemical fridge, labeled for non-food use, solves far more issues.
Container Choice: Don’t Skimp Here
Those flimsy plastic containers from the hardware store won't protect special ionic liquids. Over the years, glass vials with Teflon-lined caps do a better job. They seal tight enough to stop leaks and block the tricks humidity tries to play. Any sign of residue around the cap, swap containers. A careless transfer can destroy expensive product, send fumes into the air, and lead to a day of deep cleaning nobody wants. For long-term storage, dark glass stops light from breaking down the compound, keeping everything inside reliable.
Labels and Tracking: Protecting Your Team
Expired tags or missing dates don’t just frustrate inventory clerks—they can turn a safe space into a guessing game. I once found two containers in the same fridge: both looked identical, both labeled “N-Methylimidazolium Trifluoroacetate,” but only one had the right date and batch number. One mistake would have thrown off a week’s worth of syntheses. Barcodes, strong pen ink, and digital logs beat sticky notes every time. Safety data sheets should sit within arm’s reach, not buried in a drawer.
Accidental Spills: Be Ready Every Day
Every lab experiences a spill at some point. Quick clean-up matters far more than denial, and so does prevention. Lab coats, gloves, and goggles stay on until the last pipette is cleaned and the fridge doors are closed. Easy access to spill kits stocked with absorbent pads and pH-neutralizing agents makes sure small accidents stay small. Simply mopping up with paper towels can leave behind unseen contamination and degrade future work.
Training Makes Safety Stick
New researchers often assume someone else will tell them the right way to handle chemicals. In truth, everyone benefits from clear walkthroughs and repeated drills. Voice of experience counts—a quick demonstration of sealing, labeling, and storing beats a page of email instructions. I remember my first oversight: leaving a salt on the bench for “just a minute” brought down a whole synthesis run. That lesson stuck.
Smart Systems, Not Just Smart People
Robust storage routines help keep every bottle safe for the next user. Automated reminders to check seals, regular audits of expiry dates, and group safety reviews all help. In my experience, shared responsibility beats finger-pointing when things go wrong. Safety, after all, grows out of habit and teamwork, not strict rules alone.
Packing the Details: Understanding Purity Levels
In a regular laboratory, everyone wants to trust the bottle labels. N-Methylimidazolium trifluoroacetate – not as common as table salt, but if you’ve handled ionic liquids or specialty reagents, you've probably paused to check the fine print on its spec sheet. Most suppliers list the standard purity at or above 98%. Some labs demand better, chasing after 99% or higher. It’s not a number for show. Even a few stray impurities cause unwanted side reactions, mess with physical properties, or worst of all, derail expensive syntheses.
From my time in research, it was common to request analysis certificates alongside the purchase. These typically show moisture content (sometimes below 0.5%), halide traces, and residual solvents. Traces of water can ruin yield or produce side-products, especially since this ionic liquid often ends up in moisture-sensitive chemistry. Some protocols require the reagent freshly distilled, run through alumina to get rid of colored impurities, or vac-evaporated to squeeze out extra water.
What the Spec Sheet Doesn’t Say
Suppliers usually list purity, water content, and residual halide or acid levels. Yet, what’s stated isn’t always what lands on the bench. Over time, those imidazolium salts might pick up moisture from the air. It pays to store them tightly capped in a dry cabinet. The trifluoroacetate part, with its strong electron-withdrawing trifluoromethyl group, is one reason this salt matters: it brings unique solubility and reactivity, so keeping unwanted ions out is serious business.
Lab folks sometimes add their own checks. Throwing a sample into NMR or running a conductivity check in solution tells you more about what’s lurking in the background. With costly projects or pharma-scale work, even a .01% contaminant can turn a routine run into hours of troubleshooting. I’ve seen analytical teams debate over peaks in the NMR spectrum for a day, tracing them back to slight impurities in ionic liquids.
Chasing Quality: What Actually Makes a Difference?
Labs that settle for "good enough" batches, skipping on quality checks, end up gambling. A small deviation in purity changes the story—yield drops, products discolor, or unexpected byproducts show up in spectra. For research pushing boundaries, the temptation is to re-purify in-house. Preparing your own oxide-free, dried, and distilled N-methylimidazolium trifluoroacetate turns into a necessity for highly sensitive syntheses.
The best solution isn’t always buying the highest purity label, but forming a real partnership with your supplier. Push them for detailed batch data. Some give full NMR, GC-MS, or ICP-MS reports on request. Keep a log of incidents, and report oddities back. If enough clients highlight specific impurities, suppliers often tweak their purification steps.
Looking Ahead: Setting Practical Standards
Every lab could benefit from simple, no-nonsense testing protocols upon receipt—moisture checks, NMR scans, microscopic inspection for unusual crystals or color. Budget constraints force staff to compromise, but flagging sub-par batches early saves headaches in the future. To move the field forward, more open sharing about purity problems and supplier variability helps everyone. This collective feedback makes real improvements in chemical manufacturing and supply. Reliable data, careful sourcing, and vigilance give projects the edge they need to succeed.