N-Octyl Pyridinium Bromide: An In-Depth Look at a Modern Chemical Mainstay
Historical Development
Long before chemical catalogs listed N-Octyl Pyridinium Bromide, researchers chased quaternary ammonium salts for their surface-active and antimicrobial powers. Back in the 1940s, the hunt for better antiseptics prompted scientists to explore derivatives of pyridine. As the pharmaceutical and cleaning product industries grew, so did interest in materials that could deliver targeted biological action and reliable performance. Since then, chemists have refined both the synthetic methods and purity controls for N-Octyl Pyridinium Bromide, allowing for consistent results in labs and on manufacturing lines across the globe. Personal experience with chemical catalogs from the 1980s shows early listings carried scant details and uncertain purity markers—a far cry from today’s transparent, data-rich offerings.
Product Overview
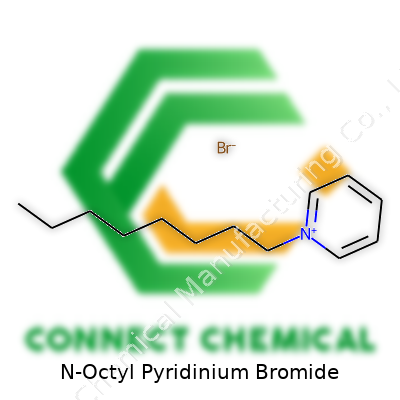
N-Octyl Pyridinium Bromide is a quaternary ammonium compound built from a pyridinium ring with an eight-carbon chain. This configuration grants the product a dual nature: a hydrophilic charged core and a significant hydrophobic tail. The market now offers both research and commercial grades, sometimes certified for GMP environments and frequently accompanied by full spectral and chromatographic profiles, giving buyers real confidence in batch-to-batch consistency.
Physical & Chemical Properties
N-Octyl Pyridinium Bromide typically shows up as a white to pale yellow crystalline powder. Under room temperature, it stays solid with a melting point reported between 180–185 °C. It dissolves easily in water, ethanol, and methanol, creating clear solutions. Its surfactant action springs from the long alkyl group, which tries to anchor itself in hydrophobic environments, while the pyridinium ring, carrying a positive charge, remains attracted to polar solvents like water. Even in high salt or alkaline conditions, solutions stay stable—a boon in both biological research and industrial cleaning processes. A robust safety data sheet summarizes its key identifiers: CAS number 10478-24-9, molecular formula C13H22BrN, formula weight 272.23 g/mol. In the lab, this information lines up neatly with real-world handling, as hygroscopicity rarely causes shelf-life issues if storage jars are closed tightly and kept away from direct sunlight.
Technical Specifications & Labeling
Labels on containers of N-Octyl Pyridinium Bromide often tell a story. Beyond purity percentages—some suppliers assure 98% or better—supporting analytical data can include gas chromatography or NMR spectra. Manufacturers print hazard statements and signal words dictated by global harmonized standards, indicating the compound’s moderate toxicity if ingested or inhaled. Containers used for shipping regularly carry secondary containment to prevent both humidity ingress and accidental spills. In laboratory circles, receiving a new supply often prompts a quick verification by melting point or TLC to check for gross contaminants, reinforcing trust in the certificate of analysis. Clear batch numbers, production dates, and storage guidance round out the labeling, nudging users to maintain compliance and traceability.
Preparation Method
Classical synthesis of N-Octyl Pyridinium Bromide usually begins with pyridine and 1-bromooctane. Stirring these together under gentle heat in a suitable solvent, perhaps acetonitrile, sparks a nucleophilic substitution: the pyridine nitrogen latches onto the octyl group, and the bromide ion balances the charge. Once the reaction’s finished, most chemists cool the mixture, prompting the product to crystallize out or letting it drop from solution after solvent removal. Multiple washes with ethyl acetate, hexane, or cold ethanol, followed by vacuum drying, push purity up and solvent levels down. Over the years, I’ve seen prep methods tweaked for better yield by controlling temperature or changing solvents, with most researchers favoring a balance between speed and minimal by-product formation.
Chemical Reactions & Modifications
The quaternary nitrogen in N-Octyl Pyridinium Bromide acts as a versatile launching platform for further chemistry. If you ask an organic synth chemist, this compound serves as a reactive surfactant or phase-transfer catalyst in nucleophilic substitution and oxidation reactions. The bromide can exchange for other halides, even for larger anions needed in material science. The octyl tail sometimes gets swapped for longer or shorter chains, or even functionalized groups, to tune solubility or biological activity. In personal lab settings, blending N-Octyl Pyridinium Bromide into formulation mixes often improves dispersion of hydrophobic components—a benefit in both polymer synthesis and dye solubilization. It sometimes pairs up with silver, copper, or other metal ions to yield antimicrobial complexes, opening new doors in coatings and medical device applications.
Synonyms & Product Names
Names for this compound change with context and supplier. You’ll see “1-Octylpyridinium Bromide” on some chemical inventories and “N-Octylpyridinium Bromide” or “Octylpyridinium Bromide” elsewhere. Some research formulations call it “OPyBr” by abbreviation, especially useful in technical documentation or on sample vials spotted in biochemistry labs. PubChem and major chemical manufacturers keep synonyms organized, which shortens time spent cross-referencing literature or regulatory documents.
Safety & Operational Standards
Working with N-Octyl Pyridinium Bromide brings certain occupational safety habits front and center. Its toxicity profile calls for gloves, goggles, and good local ventilation, especially during weighing or dissolution. The compound irritates the skin, eyes, and mucous membranes, so accidental exposure prompts quick rinsing with water and possible medical attention. In case of small spills, sweeping up with an inert binder, then sealing waste for chemical disposal, follows best practice. Researchers should never pipe by mouth (a dated but persistent risk in resource-limited settings), nor leave the material open near food areas. In my experience, regular audits and hands-on safety training prevent nearly all common mishaps in chemistry teaching labs. Material safety data sheets from reputable suppliers spell out emergency responses and handling rules, covering fire, accidental release, and disposal.
Application Area
N-Octyl Pyridinium Bromide finds diverse use in research, product development, and manufacturing. Its antimicrobial action fits formula needs for disinfectants, mouthwashes, and hand sanitizers, hitting Gram-positive and Gram-negative bacteria with equal effectiveness. The surfactant nature, paired with cationic charge, allows blending into specialty coatings and corrosion inhibitors, supporting applications in marine paints, water treatment, and oil recovery. In pharmaceutical settings, it features as a solubilizer or stabilizer, nudging active ingredients into solution or helping to control taste in oral-mucosal sprays. Practical experience shows that pilot-scale testing brings surprises—synergistic effects with other biocidal agents, or compatibility limits with sensitive biological matrices. Scientists across electrochemistry or analytical labs sometimes harness its properties to modify electrode surfaces or boost separation efficiency in capillary electrophoresis and HPLC.
Research & Development
Laboratories worldwide keep pushing the boundaries of N-Octyl Pyridinium Bromide’s capabilities. Ongoing projects track modifications in the alkyl chain, tweaks to the pyridinium ring, or pairing with novel counterions. Researchers design derivatives hoping to notch up antimicrobial activity while reducing cytotoxicity to human cells. Many universities pull in grants for studies into its role in gene delivery, as the cationic character helps bind DNA or RNA and ferry it into cells. A quick scan of recent patent filings shows commercial innovation in blending this compound into barrier films, wound care products, and dental varnishes. Academic-industry partnerships bring fresh insight to formulation stability, with staff swapping field notes on the quirks and serendipities uncovered during scale-up from bench to kilo-lab.
Toxicity Research
Toxicological data for N-Octyl Pyridinium Bromide draws attention from regulatory authorities and health scientists. Acute oral and dermal exposure studies focus on LD50 metrics in animal models, flagging moderate toxicity but avoiding alarm bells that ring for more volatile or carcinogenic surfactants. Repeated low-dose exposure sometimes prompts mild irritation or organ changes, keeping chronic exposure on the radar during inhalation or skin contact in production settings. There’s ongoing scrutiny by environmental toxicologists: the compound breaks down slowly in aquatic systems, lingering in sludge or biofilms, hinting at possible long-term risks to non-target species. My own work in a wastewater chemistry lab supported efforts to map out its pathways and partitioning, underlining the real-world need for comprehensive risk assessments on discharge and remediation.
Future Prospects
The next chapter for N-Octyl Pyridinium Bromide will depend on both regulatory trends and technical advances. Growing scrutiny over quaternary ammonium compounds’ persistence in the environment pushes green chemistry advocates to develop more biodegradable forms or improved waste treatment measures. The antimicrobial market will probably see stricter standards for toxicity and residue levels, challenging formulators to balance effectiveness with safety. New research into targeted delivery systems in medicine—using the cationic charge for selective binding or membrane penetration—hints at more specialized, higher-value products. Academic and industry collaborations may soon unlock roles in next-generation drug delivery or microfluidic device fabrication, as the need for robust, adaptable surfactants continues to rise.
The Chemistry Behind Its Power
N-Octyl Pyridinium Bromide draws attention among quaternary ammonium compounds with its reliability in disabling bacteria, fungi, and some viruses. Scientists figured out that its structure makes it especially good at breaking down microbial cell membranes, which pushes it to the front lines of infection control. It’s not just an ingredient on a safety data sheet—it’s a substance that plays a direct role in keeping everyday environments cleaner.
Disinfectants: Hospitals and Beyond
Cleaning protocols in hospitals require products that work fast and thoroughly. N-Octyl Pyridinium Bromide fits right into formulations that target hard-to-kill pathogens, including strains that resist typical treatments. Its cationic nature allows it to cling to surface grime and organic matter, disrupting microbial cells on contact. I remember reading a clinical study that showed quats outperformed other classes of disinfectants on bedrails and high-touch surfaces. The presence of this compound isn’t obvious, but nurses and custodians have trusted products spiked with it for years.
It crops up outside of hospitals too. You’ll find it in public transit sanitation, gym wipes, and cleaning sprays built for schools and restaurants, where traffic and risk go hand-in-hand. People want to know the places they frequent won’t turn into hotbeds for illness, especially after shared equipment like weights or keyboards. Manufacturers include N-Octyl Pyridinium Bromide for that added layer of reassurance and real-world effectiveness.
Oral Care: Fighting Plaque and Germs
Walk down any toothpaste aisle, and you’ll run into mouth rinses designed to target more than just bad breath. Dental professionals look to quaternary ammonium compounds for more consistent antibacterial coverage compared to older ingredients. N-Octyl Pyridinium Bromide helps control the films of bacteria that create tartar and contribute to periodontal problems. Some studies suggest regular use of such rinses reduces gingivitis markers by nearly a third over six months.
Personal care companies considered consumer safety long before regulations caught up. They keep concentrations well below irritancy thresholds because the mouth’s lining is sensitive. Emerging research now focuses on combining such agents with natural extracts, aiming to boost protection without sacrificing comfort.
Preserving Products We Rely On
Fungi and bacteria put a damper on shelf life for dozens of items, from hand lotions to industrial polishes. Brands add N-Octyl Pyridinium Bromide to slow down spoilage by targeting contaminants on the surface and within the matrix of creams or gels. This makes sense from a sustainability point of view: less waste, fewer repeat purchases, and more trust for both businesses and buyers.
During a stint at a cosmetic startup, I sat through plenty of brainstorming sessions about how to guard formulas through a humid summer, and what kept coming up: persistence. Quats like this compound helped dismiss the lingering anxiety that something might go wrong with a batch six months later.
Challenges and Future Considerations
Regulators scrutinize any substance with a broad reach in human spaces. Resistance and toxicity risks remain hot topics, making it essential for manufacturers to update formulas and rotate biocides. Science doesn’t stand still—next-gen cleaning and care products already line up alternative antimicrobials to keep ahead of nature’s curveballs.
N-Octyl Pyridinium Bromide stays part of the conversation for one simple reason: it works where common germs congregate. Relying on evidence and real-world problem-solving ensures its continued role in the products people need every day, while watchdogs and researchers keep safety and impact at the forefront.
Understanding the Chemistry Behind the Compound
N-Octyl Pyridinium Bromide stands as a quaternary ammonium salt, a family of chemicals often found in applications ranging from microbiology to surface science. Its structure features an octyl chain—an eight-carbon straight chain—grafted onto a nitrogen atom in the aromatic pyridine ring. This forms a positively charged pyridinium ion, balanced by a bromide ion. The molecular formula reads as C13H22BrN, with a molecular weight of 288.22 grams per mole.
Looking at its chemical makeup, the octyl group extends the hydrophobic tail on the molecule. This long hydrocarbon means the molecule snaps into place at interfaces, such as between oil and water, giving it practical roles as a surfactant. Chemists and biologists use this property to disrupt membranes, break down biofilms, and stabilize emulsions. In my years working with lab detergents and antimicrobial agents, I’ve seen how chain length tweaks the balance between solubility and biocidal strength. N-Octyl Pyridinium Bromide offers a good compromise: it’s bulkier than its methyl or ethyl cousins, but not so large it becomes impractical for dissolved applications.
The Weight of a Molecule: Why 288.22 Matters
Molecular weight isn’t just a number—it guides decisions in the lab every day. Knowing the exact mass helps weigh out precise amounts, especially for sensitive experiments where stoichiometry matters. For folks in pharmacy or chemistry, using the right molecular weight supports safe dosing and accurate results. N-Octyl Pyridinium Bromide’s 288.22 g/mol allows for easy handling and measuring, whether setting up a buffer for DNA extraction or adding a surfactant to a microbiological growth medium. This number also helps calculate concentrations for industrial cleaners and health-related products.
Not Just Science: Real-Life Uses and Concerns
The presence of both a hydrophobic tail and a charged head gives N-Octyl Pyridinium Bromide power as a disinfectant and surfactant. I remember training students on surface disinfection—quaternary ammonium compounds reliably kill bacteria by poking holes in their membranes. Yet there’s a responsibility that comes with these features. Persistent use of such chemicals sometimes creates resistant strains or poses hazards to aquatic life when waste gets washed down the drain. Those of us in research or product development weigh these risks, pushing for testing that ensures the benefits don’t get overshadowed by environmental harm. Studies suggest that longer alkyl chains on such compounds may cause more bioaccumulation, so environmental safety evaluations stay a top priority.
Paving the Way for Safer Chemistry
Finding the right balance between effectiveness and safety stands as a constant challenge for the chemical industry. N-Octyl Pyridinium Bromide fits in well for medical and cleaning applications due to its potent surface activity. To manage potential risks, some labs explore greener alternatives and more biodegradable formulations. Collaboration between researchers, industry experts, and environmental agencies steers the conversation in a smarter direction. This way, people benefit from the cleaning and antimicrobial action, while nature gets some protection too.
Everyday Steps that Keep the Lab Safe
I remember early days working in a research lab, juggling glassware and chemical bottles. Back then, I thought reading a safety data sheet was enough. Some of us picked up habits by watching senior colleagues. Looking back, I realize paying attention to details is what kept the accidents away. N-Octyl Pyridinium Bromide carries a special set of risks. This chemical isn’t just another name on a shelf. Its properties demand respect—skin can get irritated and inhaling it isn’t wise.
N-Octyl Pyridinium Bromide likes a cool, dry space away from sunlight. Sunlight—especially through an office window—can set off nasty chemical changes. Keeping it dry matters just as much. I once lost a week’s work after a batch soaked up moisture and clumped. Contamination ruins both results and budgets. Using sealed, labeled containers does more than organize a shelf; it protects everyone who comes after you.
Personal Protection is Not Just for Show
Splash-proof goggles, nitrile gloves, and lab coats rise to the top of my packing list whenever pyridinium compounds show up on my desk. Labs that cut corners on PPE send the wrong signal. A drop on bare skin brings rashes. Breathing in the dust risks inflamed airways—nobody wants to cough for days from one careless moment. I trust gloves rated for chemicals rather than the thin plastic extras. Any chemical that lists “may cause burns” in handling guides deserves that attention.
Ventilation in the Lab
Lab air tells its own story. A slight tingle at the back of the throat after weighing out N-Octyl Pyridinium Bromide signals poor airflow. Good ventilation pulls dust and fumes away before anyone notices. Fume hoods work best for weighing powders or mixing with solvents. I’ve stood in older labs where dust collected on shelves and filters barely did their job. Upgrades and regular filter checks brought air quality back, making days in the lab more pleasant and safer for everyone.
Clean-Up Means Prevention, Not Panic
Spills happen. Years ago, I watched new team members freeze at the sight of white powder across a bench. A printed spill protocol showed the way: scoop up with a spatula, dampen a towel to trap fine dust, avoid sweeping since dry dust spreads fast. Staff training turned panic into routine—everyone knew rubber gloves and proper disposal bags waited in a marked cabinet. It pays to keep the clean-up station clearly stocked, not hidden behind old boxes.
Long-Term Storage: Don’t Forget the Label
Labels fade. Permanent markers help, but nothing beats a printed tag with a clear date. I learned the hard way that a missing label forces colleagues to throw away an entire bottle rather than risk the guess. Detailed labels listing content, hazard class, and opening date keep history clear. Old stocks shouldn’t gather dust. Periodic checks for leaks or degraded material keep surprises away. Regular reviews match every bottle in storage to its logbook entry, closing the loop on traceability.
Build Habits, Not Shortcuts
N-Octyl Pyridinium Bromide stands as a clear example that safe chemical storage and handling aren’t lofty policies. They’re the sum of small, careful steps. I’ve seen seasoned chemists take five minutes extra to check lids and follow tidy storage plans. Their commitment influences everyone. Good storage practices and clear protocols mean fewer incidents, cleaner results, and peace of mind. Labs thrive not just from rules but from culture. Each bottle, each label, and each set of gloves—these actions build trust in the workplace and set an example anyone can follow.
What Does “Purity” Mean for N-Octyl Pyridinium Bromide?
Pure chemicals make a real difference in research and production. For N-Octyl Pyridinium Bromide – a compound found in labs, research work, and a handful of industrial settings – purity often draws a line between success and wasted hours. No researcher wants an experiment ruined by mysterious variables. Purity tells you how much of what’s inside the container is actually the desired substance, not leftovers from manufacturing or transport. In practice, most reputable suppliers place N-Octyl Pyridinium Bromide in one of two major purity categories: 97% and 98%. In select cases, bottles marked 99% show up, but that climb in the numbers comes at a price.
Why Do Small Differences in Purity Matter?
A percentage point or two may look minor. In practice, those small numbers have a heavy influence. Research settings, like synthetic organic chemistry or pharmaceutical work, chase after the purest material they can get their hands on. Even one percent of side products can sway results or throw off a careful synthesis. Quality assurance teams, for instance, have flagged studies where lower purity led to contaminant byproducts, changing physical properties or biological activity.
A few years back, while troubleshooting a stubborn chemical reaction, I found out just how much a so-called “minor” impurity can shape the outcome. We traced the problem to trace bromide salts left from a lesser grade of N-Octyl Pyridinium Bromide. A switch to 98% purity meant job done, plain and simple.
Checking Your Source: Not All Purity Claims Are Equal
Plenty of chemical catalogs list 97%, 98%, or “high purity” N-Octyl Pyridinium Bromide. Reading those certificates of analysis is where the truth comes out. Reliable manufacturers back up their claims with HPLC or NMR data – not just a figure tossed into a PDF. If the information remains vague, or the “certificate” repeats marketing copy, it’s time to look somewhere else.
Most famously, Sigma-Aldrich and TCI supply upward of 98% purity and display their analytical method up front. There are lesser-known vendors, but sticking with those who publish the data earns some peace of mind. In real life, choosing a transparent supplier means fewer bad surprises during research.
Paying for Purity: Worth the Extra Cost?
Higher purity brings higher prices. Some might ask if a 2% upgrade in purity is worth double the cost. For exploratory lab work or method testing, maybe 97% passes muster. Yet, in final product manufacturing or drug development, there’s little room for doubt. Extra cost up front beats spending weeks on failed reactions or discarded batches. Thinking about the time, labor, and supply chain headaches saved, it’s a pretty easy decision.
Moving Forward: How Purity Shapes Progress
Looking at the bigger picture, the pursuit of purity never stops at chemicals. Trust grows from clear data, repeatable outcomes, and honesty in those supply chains. In my own work, asking tough questions about purity never failed me. Whether it’s N-Octyl Pyridinium Bromide or another odd-sounding reagent, the purest path remains the one where no shortcuts get taken.
Understanding What’s at Stake
N-Octyl Pyridinium Bromide lands on the list of chemicals with sharp personalities. Labs and factories go for it because it acts as a disinfectant, a surfactant, and a powerful antimicrobial agent. It sounds useful, so concerns might not rise right away. But no one enjoys surprises after the fact. With chemicals like this, health and environmental questions come up fast, and for good reason.
Following the Science on Human Health
Getting a bit of this compound on your skin may cause irritation or a burning feeling. Inhaling the powder or vapors leaves your throat scratchy and lungs uncomfortable. Eyes don’t fare any better. The compound stings, and if not treated right away, it can damage tissue. Eating it by accident is much worse. Nausea, vomiting, or worse can show up, depending on the amount swallowed.
Workers handling the chemical often refer to the Safety Data Sheet (SDS). The SDS outlines that personal protective gear is not optional. Gloves, goggles, and solid ventilation cut down accidental exposure. The chemical can trigger allergic reactions too, so even a slight slip-up puts sensitive people at risk. A friend who worked in a cleaning supply lab told me he underestimated how quickly skin redness appeared, even through a lab coat sleeve.
Environmental Impact Can't Get Ignored
Factories using tons of N-Octyl Pyridinium Bromide can end up releasing some into wastewater or the environment. This stuff does not break down easily. Water plants won’t always filter it out, threatening aquatic life. Fish and aquatic insects are particularly sensitive to this class of chemicals. Too much of it in rivers or lakes pushes delicate ecosystems out of balance.
Waste disposal takes effort. Pouring leftover solution down a drain may seem harmless, but it’s short-sighted. Environmental agencies in Europe and North America label it hazardous, banning casual disposal. I’ve seen colleagues go through hours of paperwork and special drum pickups just to get rid of small amounts left over at the end of a project.
Addressing Hazards the Responsible Way
Personal safety relies on discipline. Training sessions matter. If someone handles this chemical for the first time, they need the full run-down: proper gloves, splash-proof goggles, lab coats, and ventilation. Respirators may also come into play in tight spaces. Taking shortcuts only leads to accidents, which then mean medical bills or lost work hours.
Labeling and storage are straightforward but critical pieces. Labels should be clear, not hidden behind dusty jars. Locked chemical cabinets prevent mistakes and limit who can reach it. Using spill kits and eye wash stations, tested regularly and placed near working areas, cuts emergency times. Cohesive planning outside of the textbook—the stuff learned from routine practice—does miles more than any fancy new safety poster.
Searching for Better Approaches
For all its utility, this chemical sits among several choices. Researchers and manufacturers benefit from regular safety reviews. Finding gentler alternatives might not always be simple, but health and environmental costs tip the balance. Companies can run pilot tests with newer, less irritating disinfectants and document how well they clean without the baggage.
Chemicals like N-Octyl Pyridinium Bromide always command respect. Clear training, transparent labeling, thoughtful disposal, and real investment in safer substitutes carry more weight than any short-term gains. People and the planet both pay the price when we cut corners.