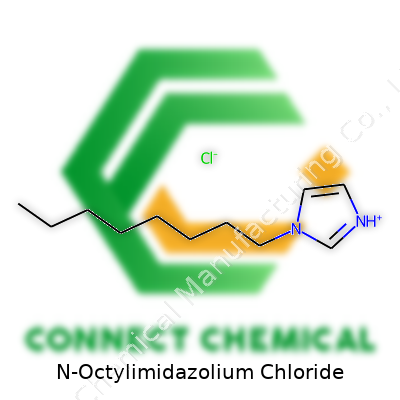
N-Octylimidazolium Chloride: A Down-to-Earth Commentary
Historical Development
N-Octylimidazolium chloride grew out of the massive interest in ionic liquids that swept the chemistry world in the late twentieth century. Researchers saw imidazolium salts as game-changers for green chemistry and began tinkering with longer alkyl groups to fine-tune stability, solubility, and handling properties. The idea of sticking an octyl chain onto the nitrogen atom was not just a question of improving oil solubility; it also changed how the molecule interacted with both organic and inorganic reagents. Like a lot of tools in a chemist’s toolbox, N-Octylimidazolium chloride didn’t pop onto the scene overnight. Its story is tied to the growing realization that conventional solvents were taking a serious toll on the environment and people’s health. As new ionic liquids came off the bench, the imidazolium core kept proving itself. People started talking about this stuff in journals and at conferences, eager to see if it could help industries move past the stubborn reliance on volatile organics.
Product Overview
N-Octylimidazolium chloride belongs to the family of quaternized imidazolium compounds. The molecule itself brings together an aromatic imidazole ring and a straight, eight-carbon alkyl group, capped off with a chloride anion. Commercial suppliers offer it as a white to off-white powder or sometimes as a viscous liquid, depending on purity and dryness. Its unique molecular arrangement pulls together hydrophobic and hydrophilic domains, which means it likes to play with both polar and non-polar molecules — a rare trait in industrial chemistry. Unlike stuffy legacy solvents, N-Octylimidazolium chloride won’t vanish into the atmosphere. Its vapor pressure stays low even at elevated temperatures, cutting down on emissions and health risks.
Physical & Chemical Properties
This compound often lands on lab benches as a stable, crystalline powder. Its melting point usually sits above room temperature, although batch purity or trapped water can nudge it up or down. The octyl chain renders the substance less water-soluble than its methyl or ethyl cousins, but the chloride portion ensures reasonable dissolving in alcohols and other polar organic solvents. Folks appreciate that it won’t break down or corrode common lab materials. Chemically, the chloride anion participates in hydrogen bonding while the imidazolium cation engages in π-π stacking or hydrophobic interactions, depending on the reaction partners. Its thermal stability reaches above 200°C before decomposition, so it's handy in elevated-temperature synthetic routes.
Technical Specifications & Labeling
Quality control in chemical supply always deserves respect. Labs and manufacturers label each batch of N-Octylimidazolium chloride with its purity, water content, and trace metals, since even minor impurities skew sensitive applications. Typically, high-end lots run above 98% purity, barring solvents, unreacted starting materials, or halide contaminants. Detailed safety data sheets accompany each shipment, breaking down hazards and shelf-life expectations. The chemical goes out in double-sealed containers because moisture and stray acids can degrade the cargo in transit. Clear batch numbers on the label let users trace any trouble back to the production date or synthetic route.
Preparation Method
Most syntheses start by reacting 1-methylimidazole with 1-chlorooctane in a polar solvent like acetonitrile, under a nitrogen blanket and with steady stirring. After dozens of hours at mild heat, the mixture usually separates into two layers. Chemists extract the bottom layer and recrystallize it from ethanol or acetone to clear out leftover reactants. The resulting white powder passes through charcoal and vacuum filtration steps to lock down purity. Large-scale operations automate much of the process, swapping out glassware for stainless steel reactors and controlling temps and nitrogen feeds by computer. Scale-up means the equipment must handle corrosive byproducts and withstand long continuous syntheses without pitting, so vendors often work closely with chemical engineers on design.
Chemical Reactions & Modifications
N-Octylimidazolium chloride plays an active role in organocatalysis, phase-transfer catalysis, and materials chemistry. The imidazolium portion enables cationic exchange with other halides, which means swapping chloride with larger or more complex anions at the snap of a finger. The octyl group can undergo oxidation to yield hydroxylated versions, giving the molecule even more grip on polar reactants. One underrated reaction: the quaternary nitrogen can open doors to nucleophilic substitution pathways you won’t see in more pedestrian ionic liquids. In both lab and plant settings, users often tailor the molecule through metathesis, kicking out the chloride for something more compatible with a specific target reaction. Some researchers even tag the compound with fluorophores or doping metals, expanding its role as a specialty reagent in nanoscience or sensors.
Synonyms & Product Names
In scientific papers and supplier catalogs, N-Octylimidazolium chloride shows up under a stack of names. You’ll spot it listed as 1-Octyl-3-methylimidazolium chloride, OMIM-Cl, or sometimes just “octylimidazolium salt” in casual conversation. Each synonym reflects the same core: an imidazolium ring, a straight eight-carbon arm, and a chloride anchor. Savvy chemists check the exact substitution pattern — improper cataloging sometimes spawns confusion with analogs like benzyl derivatives or short-chain substitutions. Correct naming clears up any headaches in ordering, record-keeping, and experimental reporting.
Safety & Operational Standards
Handling N-Octylimidazolium chloride means showing it some respect. Unlike highly volatile solvents, it won’t gas you out of the lab in seconds, but skin and lung contact can wind up causing irritation or sensitization. Most practitioners rely on gloves, splash goggles, and bench hoods, even for short syntheses. In my own lab days, I saw a few careless students ditch the gloves after forgetting about delayed skin reactions; it wasn’t worth the risk. Waste handling follows strict protocols—liquid and solid residues need containment in halogenated waste streams and shouldn’t ever meet sewer pipes or general trash bins. Because it’s not readily biodegradable, regulators in many countries treat it just as seriously as classic industrial chemicals, so training and compliance audits remain routine.
Application Area
Real world uses for N-Octylimidazolium chloride stretch across several industries. It gives metal extraction processes a leg up by acting as a ionic liquid phase for selective leaching or purification. Analytical labs lean on it as a mobile phase additive in liquid chromatography, boosting separation between closely related compounds. I’ve talked to fellow researchers who found it stabilized colloidal nanoparticles better than many surfactants, thanks to that long octyl tail. Other folks drop it directly into catalytic reactors, exploiting its stability at harsh temperatures and harmlessness towards delicate substrates. Electrochemical setups value its broad electrochemical window, which means researchers can push batteries, capacitors, or sensors farther without seeing breakdown or interference.
Research & Development
Researchers keep finding creative twists for this chemical. Environmental scientists dig into how its unique solubility profile separates heavy metals or dissolved organics from wastewater. Pharmaceutical teams run compatibility studies for drug delivery using N-Octylimidazolium chloride as a solubilizer or co-crystallization partner. Polymer chemists graft the imidazolium core onto polymer backbones, aiming to make new ion-conductive films or membranes for clean energy tech. I remember hitting a wall in a collaborative project until we subbed in the octylimidazolium salt and suddenly started seeing promising activity in our supported catalyst system. Open-ended funding lets people try risky experiments, and N-Octylimidazolium chloride keeps up with those high demands for both versatility and safety.
Toxicity Research
Toxicologists note that ionic liquids carry much lower vapor risks than chloroform, ethers, or old-school halide solvents, but they don’t give N-Octylimidazolium chloride a total pass. Animal studies highlight mild to moderate toxicity; liver and kidney effects have shown up in some rodent models after repeated high-dose exposures. Cell assays also show cytotoxicity above certain concentrations, so labs take care mixing and weighing powder. It doesn’t seem to persist or bioaccumulate in animal tissues as aggressively as some perfluorinated organics, but regulators still flag the need for waste collection and safer disposal. Environmental questions persist — the octyl side chain can resist easy bacterial degradation, so research continues on making its byproducts less persistent.
Future Prospects
Looking forward, N-Octylimidazolium chloride’s story has hardly run its course. Industrial chemists eye its use in battery electrolytes and new fuel cell membranes thanks to its chemical and thermal stability. There’s promising startup work happening now, betting this ionic liquid can cut costs and environmental harm across a load of extraction and separation steps. On the academic side, bio-inspired modifications to the side chain or counter-ion might create new hybrid materials or medical imaging agents. It feels like chemists have just started scratching the surface, and each new application brings a fresh batch of real-world problems for the molecule to solve. As environmental rules get tighter, compounds that check both performance and safety boxes — like this one — have a shot at becoming tomorrow’s gold standard.
Understanding Where It Fits In
N-Octylimidazolium chloride slips into labs and factories for jobs that call for a tough worker with a unique set of skills. The chemistry world calls it an ionic liquid—a type of salt that stays liquid at much lower temperatures than folks would expect. It mixes its talent for dissolving things with a knack for splitting up organic and inorganic molecules that don’t like to play together. That means researchers reach for this stuff in all sorts of new technologies, like fuel cells or greener ways to clean up chemical waste.
How Chemists Actually Use It
In my grad school days, tinkering in the lab, everyone stayed curious about materials that could actually make a shift toward greener chemistry. N-Octylimidazolium chloride made frequent appearances when we needed safer substitutes for toxic solvents. It’d dissolve metals, separate out dyes, or pull rare molecules from water that nothing else would touch. The point wasn’t just to solve technical puzzles—it meant fewer hazardous chemicals down the sink, less need for expensive ventilation, and a few less headaches by the end of the day.
Beyond the lab, factories have started to catch on. Pick almost any industry—electronics, pharmaceuticals, or even battery manufacturers—and you can find a use. For instance, folks building new types of lithium-ion batteries use ionic liquids like these to control conductivity and stability. That doesn’t just bump up battery life a little; it’s about avoiding toxic leaks and corrosion. Chemists and engineers use it for extracting metals from ores without blasting through tons of harsh acids. This turns what used to be nasty waste into something they can actually recycle or reuse.
Bigger Implications for Sustainability
Toxic solvents stick around in the environment. Cleaning them up drains money and impacts health in communities working near chemical plants. Ionic liquids like N-Octylimidazolium chloride tend to be non-volatile, so you don’t get the strong fumes or risk the same kind of air pollution. That doesn’t mean every ionic liquid is totally harmless; we still need long-term research on how these new chemicals behave outside the lab. But as someone who’s worked with both old-school and newer chemistries, I’ve seen what even small steps toward safer materials can mean. Fewer spills, fewer mistakes, and, plain and simple, less danger.
Looking Toward Practical Solutions
The chemical industry often hesitates to shift away from what’s familiar. Changes cost time, retraining, and investment in equipment. Some companies still resist adopting alternatives because regulations lag or costs look steep. What actually pushes things forward? Clear evidence that newer materials like N-Octylimidazolium chloride can streamline processes, cut energy spending, and reduce waste headaches. In the lab, data supporting reduced emissions and improved product recovery makes a real difference at the decision-making table.
Public accountability makes a mark here. Communities want safer air and water. Customers expect cleaner manufacturing. Companies that get ahead on safer chemicals stand to reap reputational rewards, not just regulatory compliance. Researchers—myself included—often push for collaborative studies so everyone benefits from safer, cleaner chemistry. That means more universities linking up with local industries, sharing results, and lowering barriers to adoption.
Why It Matters in Everyday Life
At the end of the day, safe chemistry isn’t just a lab or boardroom concern. Small tweaks in chemical processes echo through to the air we breathe and the water we drink. From electronics to medicine to the next-generation batteries in our homes, the shift toward chemicals with lower risks and greater utility ties in with stronger health outcomes. N-Octylimidazolium chloride shows that boring changes in what goes on behind factory doors can have an outsized impact on well-being, safety, and trust in the products we use every day.
Understanding the Risks
Anyone who has worked in a chemical lab knows the safe way to handle new substances starts with a real respect for what’s on the label—especially when it comes to compounds like N-Octylimidazolium Chloride. These ionic liquids open a world of possibilities in catalysis, extraction, and electrochemical work, but you should never let routine make you careless. Even a seasoned chemist can remember times when small lapses ended with a skin rash or a sore throat.
Personal Protective Equipment Matters
Experience in the lab tells you not to skimp on gloves or eye protection. Latex or nitrile gloves block direct skin contact. Safety goggles do their job if you tighten the strap. A lab coat keeps splashes away from your clothes and skin, but many forget about the value of closed-toed shoes until they see spillage hit the floor. N-Octylimidazolium Chloride isn’t a household chemical, and the best PPE adds a key layer between you and long-term problems.
Ventilation Keeps the Air Safe
Rooms without airflow turn harmless projects into real hazards. Running fume hoods while opening or measuring N-Octylimidazolium Chloride can protect your lungs. This isn’t just about comfort; studies have linked ionic liquids to respiratory irritation after accidental inhalation. Nobody wants to cough through a shift—turning on a hood is quicker than dealing with headaches or sore throats later.
Safe Storage Means Fewer Surprises
One lesson labs learn fast: chemicals left out don’t stay safe for long. N-Octylimidazolium Chloride needs a clearly labeled, tightly sealed container. Anyone who has cleaned up a spill knows why shelving matters. Keeping it away from direct sunlight and moisture reduces unwanted reactions. Docs from manufacturers flag both reactivity and degradation with water—it's smart to trust the people who made it.
Quick Cleanup Prevents Long-Term Problems
Spills happen. The real danger comes from letting them sit. My own experience says always having spill kits around brings peace of mind. Granular absorbents soak up liquid; paper towels make things worse if they spread it. Labs with easy access to eyewash stations and emergency showers always come out ahead when someone makes a mistake. Unprepared workspaces never forget their first spill.
Training Brings Everyone Up to Speed
You can always spot labs that take safety training seriously. People know the difference between a minor mishap and a crisis because they practice. Explaining the hazards to newcomers, reviewing SDS information, and running through emergency routines saves real pain down the road. Nobody benefits when one person knows the drill and the rest freeze up.
Disposal Shouldn't Be an Afterthought
Experienced labs keep hazardous waste bins handy. Pouring N-Octylimidazolium Chloride down a sink or tossing it in the trash sometimes tempts folks looking to save time, but local laws say otherwise for good reason. Ionic liquids persist in the environment, and letting them into water streams can create ecological headaches. Accredited waste contractors know exactly where leftover material belongs.
Building a Safe Work Environment
Working with N-Octylimidazolium Chloride rewards careful habits. Over the years, seeing both good and bad outcomes points to a single truth: the best safety procedures come from expecting accidents and still being ready for them. Regular safety audits, clear communication, and a bit of pride in your workplace do more to protect people than any written rulebook.
The Building Blocks Behind N-Octylimidazolium Chloride
N-Octylimidazolium chloride belongs to a group of chemicals called ionic liquids. The “imidazolium” bit tells me that the core structure looks like an imidazole ring. Basically, this ring combines three carbon atoms and two nitrogens in a specific arrangement called a five-membered ring. From my laboratory experience, that ring helps create a stable framework, and chemists often use it because it can be modified pretty easily.
N-Octylimidazolium chloride takes this basic imidazole ring and attaches an octyl chain, which is eight carbon atoms in a row. This gives the molecule some clout when it mixes with oils or non-polar solvents. The octyl chain replaces a hydrogen atom on the nitrogen of the imidazolium ring, which makes the molecule much more hydrophobic than its parent compound. The chloride part is straightforward—a single chloride anion balances the charge from the positive imidazolium head.
Why Structure Shapes Performance
I’ve worked with a variety of ionic liquids, and the structure of N-octylimidazolium chloride shows clear benefits in tasks like solvent separation or as an electrolyte. The long octyl group gives it lower melting points and good thermal stability, which has practical impacts. For example, if you try to dissolve different plastics or catalyze reactions in the lab, this structure often outperforms shorter chains or other salts.
The straight-chain octyl tail brings flexibility that shorter alkyl chains can’t provide. This has turned out valuable in fields like green chemistry. Since the molecule consists of a charged imidazolium ring paired with a bulky, oil-loving chain, you end up using less dangerous organic solvents. Recent studies (like those from ACS Sustainable Chemistry & Engineering) confirm that imidazolium-based ionic liquids cut down on hazardous effluent during chemical processes.
Identifying Solutions for Safer Chemical Use
I’ve always found laboratory safety a sticking point, especially with volatile organic solvents. The use of N-octylimidazolium chloride gives industries a shot at better environmental compliance. Since it hardly evaporates, the risk of inhalation drops. The imidazolium backbone also resists breaking down in the presence of strong acids or bases, which translates to less handling concern.
Researchers at multiple institutions have shown that tweaking the alkyl chain length influences toxicity and biodegradability. As a middle-length alkyl chain, the octyl version strikes a balance—it dissolves tough organic stuff without being as persistent as some longer chains. The chloride counterion isn’t particularly reactive, adding another layer of safety during storage and use.
Room for Optimization
Scalability keeps coming up in discussions around ionic liquids. The synthesis of N-octylimidazolium chloride isn’t overly complex, but the purification step often bugs operators, mainly due to leftover imidazole and by-products. I see process chemists focusing on greener purification methods—membrane separation and supercritical CO2 extraction, for instance—that help cut costs and limit waste.
Overall, the chemistry behind N-octylimidazolium chloride points to a modern way forward for materials science, catalysis, and safer industrial methods. The structure tells the story; it’s up to us to use these insights for cleaner, smarter manufacturing and research.
Sticking to Basics in Chemical Storage
Anyone who spends time in a chemistry lab or works with specialty chemicals learns quickly that good habits with storage pay off in safety, product quality, and regulatory headaches avoided. N-Octylimidazolium chloride isn’t an unfamiliar name these days in solvent development and ionic liquid research. Like a lot of imidazolium-based salts, it can react when exposed to moisture or certain metals. You don’t want to toss it on a standard shelf and call it done.
Why Moisture Protection Matters
One of the big takeaways from working with imidazolium salts is that water finds its way in if you let it. N-Octylimidazolium chloride absorbs water from the air—hygroscopic, as we say—and as that water builds up, you can see clumping or degradation. The result: unreliable results in the lab and wasted money. That’s reason enough to use airtight containers. Glass bottles with screw-threaded caps and a solid gasket do the job. Polyethylene or polypropylene containers won’t give off leachables, but glass still beats them for longer-term storage. Toss in some desiccant packs; silica gel or molecular sieves both work. You spot the blue beads turning pink—time for a change.
Avoiding Heat and Light Damage
Heat does more than just threaten decomposition. High temperatures create humidity shifts inside containers that pull in air every time it gets opened. You want to store N-Octylimidazolium chloride in a cool room, ideally below 25°C. Temperature spikes above that, especially in summer or near heat-emitting equipment, make those unwanted reactions more likely. Fluorescent lights or sunlight can also degrade some organic salts over time, so I make a point of keeping sensitive bottles away from window sills or busy benches.
Safe Distancing from Reactive Materials
Some of the worst accidents with chemicals happen when storage mixes up incompatible groups. Never keep N-Octylimidazolium chloride close to strong oxidizing agents, acids, or alkali metals. Shelf labels, color-coded bins, and properly indexed inventory take a bit of time to set up but they stop a cascade of problems. If you’ve ever had to deal with a chemical spill due to container corrosion, you remember the mess. Regular checks to look for any color change, odd smells, or caked-on residue catch problems early.
Regulatory Steps and Documentation
Depending on the workplace, safety data sheets (SDS) must be handy. Inspectors don’t want to see missing or expired sheets. An up-to-date inventory, labeled containers with hazard pictograms, and spill kits ready for use make things smoother in case of audits. Labels with batch number and date of arrival help track shelf life. Even though the chloride salt isn’t explosive or acutely toxic, it pays not to underestimate what overlooked paperwork costs in a business.
Building Safer Storage Culture
I’ve watched plenty of beginners toss chemical jars in random drawers. A better way starts with regular training and a clear map of storage zones in the lab or storeroom. Sharing case stories of what went wrong before, not just reading rules, helps people care about getting it right next time. Good chemical storage lets research and production hum along, free from the avoidable headaches poor storage can create.
Understanding Purity in Lab Chemicals
Working in a chemistry lab, few things get more attention than the purity of chemicals. Researchers often ask about the specification of N-Octylimidazolium Chloride before starting any test or synthesis. This isn't just fussiness. Purity influences the outcome of experiments, the reproducibility of results, and even lab safety. In practice, it means fewer surprises during reactions and more reliable results.
Specification Standards: What Matters Most
Companies usually offer N-Octylimidazolium Chloride at a purity level above 98%. For those unfamiliar, 98% purity means out of 100 grams, at least 98 grams will be the intended compound with about 2 grams of everything else—residual solvents, synthesis byproducts, or trace moisture. Even small amounts of impurities carry weight in sensitive applications like catalysis or ionic liquid research. The impurity profile isn't just a technicality; it draws a line between consistent performance and frustration.
Manufacturers supply a Certificate of Analysis with each batch, detailing purity by high-performance liquid chromatography (HPLC) or nuclear magnetic resonance (NMR). This certificate is not just a sheet of numbers. It reflects months of honing the production process, ensuring each shipment meets the advertised standard. Most labs rely on this documentation before committing to a supplier, especially in regulated industries. Over time, a habit of cross-referencing certificates with personal test results grows. The small print—trace sodium, residual water, halide content—matters as much as the headline purity.
Why Purity Directly Affects Outcomes
Trying to replicate a study or scale up a process with lower-purity chemicals often backfires. An extra percent of water or unreacted starting material can throw off reaction yields or product profiles. That's a headache for graduate students and process engineers alike. In catalysis projects, the smallest impurities may poison metal catalysts or prompt side reactions. Small variances turn repeatable science into guesswork. That’s not just my opinion; journal articles and industrial case studies are peppered with projects derailed by an overlooked impurity.
Real-World Impacts and Solutions
Consistency is king. Research groups should test new lots of N-Octylimidazolium Chloride with their own quality checks, just as food service professionals taste before serving. Water content can be determined by Karl Fischer titration; halide contamination by titrimetric or ion-exchange methods. Relying solely on a supplier’s stated purity carries risk, particularly in fields where a trace contaminant can invalidate months of work.
If stricter purity is required, some labs purify the material further by recrystallization or drying under vacuum. This adds cost and time, but it gives control. Open dialogue with suppliers can also help, since custom synthesis or higher-purity options are often available for an added fee. Considering the cost of failed experiments, sometimes the premium pays off.
Moving Forward
Every chemical has its quirks, and N-Octylimidazolium Chloride is no exception. From personal experience, taking purity at face value rarely ends well in complex research. Chemical suppliers carry a responsibility to publish transparent, detailed specifications, while researchers handle verification and, when necessary, further purification. The quality of research often rises or falls with that last percentage point nobody sees at first glance. That’s the reality in the lab—and what determines which projects move forward and which get stuck.