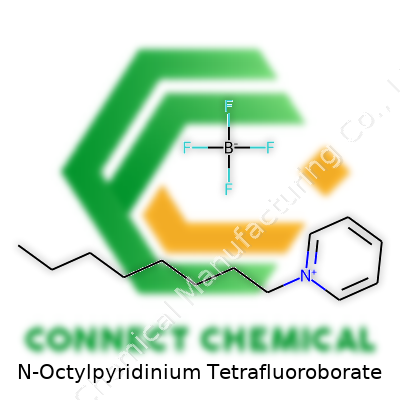
N-Octylpyridinium Tetrafluoroborate: Chemistry, Application, and Research Landscape
Historical Development
The roots of pyridinium-based compounds stretch back to early 20th-century experiments as chemists chased after new ionic materials. Interest took off as researchers realized these salts could punch above their weight in electrochemistry labs and industrial settings. N-Octylpyridinium Tetrafluoroborate came into sharper focus once chemists started tinkering with tailored ionic liquids for applications beyond traditional organic salts. Publications dating back to the 1980s flagged its emerging role as an alternative electrolyte and phase transfer agent. The story of this compound mirrors broader growth in the field of ionic liquids: a search for less volatile, more chemically robust alternatives to conventional solvents and reagents.
Product Overview
N-Octylpyridinium Tetrafluoroborate belongs to the family of ionic liquid salts, with distinct features separating it from classic alkali or ammonium salts. Its chemical framework—an eight-carbon alkyl chain attached to a nitrogen in the pyridine ring, paired with the tetrafluoroborate anion—unlocks uses ranging from advanced batteries to organic synthesis. Most commonly, researchers pick it up as an off-white to pale yellow crystalline solid or viscous liquid. Producers in the specialty chemicals market pack it by the gram or kilo, tailored for laboratories or pilot-scale research.
Physical & Chemical Properties
This compound stands out because of its good thermal stability and strong ionic conductivity. Not much smells noticeable from it—rare for a pyridinium. It dissolves well in polar organic solvents and endures under heat up to about 200°C before breaking down. A melting point typically hovers around 100–120°C, keeping it stable under room conditions but nimble enough for melt mixing in experimental setups. The octyl chain gives it more surfactant-like behavior, which can help in catalysis or extraction, while tetrafluoroborate brings chemical robustness, resisting hydrolysis better than many other ionic partners.
Technical Specifications & Labeling
Transparent technical sheets break down quality benchmarks for buyers: purity usually exceeds 98%, and water content falls below 0.5%. Detailed labeling points out molecular formula (C13H21BF4N), molar mass (295.12 g/mol), batch number, expiration, and shelf-life details. Experienced lab managers double-check certifications and spectroscopy results—chiefly NMR and HPLC—to avoid purity pitfalls that trip up sensitive experiments. The best suppliers toss in full MSDS and hazard labels per GHS standards, helping users avoid surprises under the hood.
Preparation Method
Synthesis starts with N-octylpyridinium bromide or chloride, produced through the quaternization of pyridine with 1-bromooctane or 1-chlorooctane. This intermediate reacts with sodium tetrafluoroborate during a salt metathesis. Layers separate in the right solvent, the tetrafluoroborate version lands in the organic phase, and the less useful salt stays in water. A few filtration and washing steps draw out impurities and leave a cleaner product. Rotary evaporation or vacuum drying firms up the crystalline salt, ready for reactivity tests or loading into a reactor. Reliable chemists know the order and timing of these steps spells the difference between a pure batch and a problematic one that clogs up analytic instruments or imparts trace metal contamination.
Chemical Reactions & Modifications
N-Octylpyridinium Tetrafluoroborate flexes as a versatile cation. Chemists often test its metathesis with other anions—PF6–, Cl–, or NTf2–—to alter solubility profiles or optimize catalyst turnover in new reactions. The alkyl group stays stable in most chemical spaces, but nucleophilic attack or radical processes can cleave the octyl chain under harsher protocols. In electrosynthesis, switching the counter-ion or tinkering with the alkyl tail can fine-tune oxidation potential. Many academic and industrial groups race to find more sustainable methods for swapping anions without generating toxic waste or hazardous byproducts.
Synonyms & Product Names
Catalogs and research reports list this compound under various synonyms: 1-octylpyridinium tetrafluoroborate, N-octylpyridinium boron tetrafluoride, or just OPy BF4. Misspellings sometimes crop up because of the unusual combination. CAS numbers and manufacturer-specific codes usually clear confusion in procurement circles. Big names in specialty chemical distribution, from TCI to Alfa Aesar, tag it with slight variations. Research teams working on scale-up or cross-lab projects have to verify each incoming shipment carefully; incorrect naming can slow down projects or lead to expensive reruns of key experiments.
Safety & Operational Standards
Handling pyridinium salts raises fewer alarms than many fluorinated compounds, but smart labs don’t ignore standard chemical hygiene. Direct skin contact or inhalation can irritate. Some studies flag moderate aquatic toxicity from tetrafluoroborates, guiding disposal practices. No one wants to risk accidental hydrolysis that releases HF gas. Personal protective gear—gloves, eye protection, efficient fume hoods—remains non-negotiable. Training for storage and emergency measures earns extra scrutiny, as some warehouse accidents still draw investigations after improper packaging or temperature excursions. Regulations in North America and Europe put the onus on operators to document every step, from receipt to final waste management.
Application Area
Most buyers focus on N-Octylpyridinium Tetrafluoroborate’s uses in ionic liquid development, especially as a supporting electrolyte in electrochemistry, redox-mediated transformations, or membrane research. Engineers exploring advanced batteries and supercapacitors appreciate the stable conductivity it brings. Its surfactant properties nudge it into extraction chemistry and phase transfer catalysis, making separation processes more efficient. Chemical technicians experimenting with asymmetric catalysis or organometallic systems include it as a tunable co-catalyst to boost energy efficiency. The pharmaceutical sector keeps an eye on its potential for green chemistry, where conventional solvents have grown too hazardous or emissions-heavy for modern production lines.
Research & Development
Active research circles peg N-Octylpyridinium Tetrafluoroborate as a candidate for environmentally friendlier processes and emerging electrochemical devices. Academics try novel combinations with transition metal complexes for hybrid electrolyte solutions. Pilot studies in green extraction methods draw on its ability to shuttle hydrophobic and hydrophilic materials across phases. Multinational chemistry consortiums fund trials measuring recycling efficiency and long-term stability across repeated cycles in flow cells and fuel cells. University labs hunt ways to synthesize it more cleanly, paying attention to energy inputs, reagent hazards, and scaling pitfalls. Open data on performance benchmarks and real-world case studies continue to drive adoption in the broader research community.
Toxicity Research
Toxicological studies flagged the moderate cytotoxicity of pyridinium salts, usually via membrane disruption in cells. This compound lands in the “treat with respect” camp, since excessive exposure can affect aquatic organisms and possibly bioaccumulate. The tetrafluoroborate component calls for careful disposal—no one wants boron or fluorine slipping unnoticed into water streams. Regulatory boards have watched these compounds closely, urging better labeling and proactive spill response. Academic papers also stress no evidence of carcinogenicity or severe chronic effects in standard handling, but researchers stay cautious and prefer to run toxicity screens for any new biological or environmental exposure.
Future Prospects
There’s no denying the push for cleaner, safer specialty chemicals in industrial chemistry, and N-Octylpyridinium Tetrafluoroborate finds itself at a crossroads as labs move away from toxic or unsustainable reagents. Expect more interest in its application for battery technologies, green synthesis, and phase transfer systems as the cost of traditional ionic liquids rises and regulatory pressure mounts. Research will center on making synthesis greener and better understanding long-range environmental impact. Startups and research consortia keep scanning for better cation-anion pairs, but this salt’s blend of stability, solubility, and tunability places it on the short list for future tech. Each development stage will test its place among the next generation of ionic materials, battery electrolytes, and catalytic systems.
Playing a Role Behind the Scenes
Most folks won’t spot the name N-Octylpyridinium Tetrafluoroborate in everyday headlines. On paper, it looks like yet another tongue-twister from a chemistry class. In real life, this compound carries more weight, especially for anyone that walks the line between research and practical solutions.
I remember my first brush with specialty salts came during a hands-on project in a college chemistry lab. We tried out ionic liquids to help separate tricky mixtures. That learning moment stuck with me. Scientists keep reaching for compounds like N-Octylpyridinium Tetrafluoroborate not just because it dissolves well, but because it brings unique chemical flexibility to the table.
What’s It Good For, Anyway?
Unlike household chemicals, you won’t find this one under the sink. N-Octylpyridinium Tetrafluoroborate shines in laboratories. Researchers know it as an ionic liquid: meaning, it stays liquid at lower temperatures, and its unique structure allows all kinds of fine-tuned experiments. It shows up in organic synthesis and electrochemistry projects where stable, non-volatile substances make life easier for chemists.
In my graduate program, the compound kept popping up in articles about “green chemistry.” Many industrial solvents give off harsh fumes and spark worries about health and contamination. N-Octylpyridinium Tetrafluoroborate doesn’t release those kinds of vapors, making it kinder to the environment and to the folks handling it. Reports published by specialists at the American Chemical Society point to its lower volatility and chemical stability as big reasons for ongoing interest.
Another area: electrochemical devices. Batteries and solar cells keep requiring electrolytes that stand up to long-term use and store energy efficiently. Companies working on next-generation batteries pay attention to ionic liquids like this because of their broad electrochemical windows and good thermal properties. If you keep hearing about solid-state batteries promising safer electric cars, materials like N-Octylpyridinium Tetrafluoroborate help lay the groundwork.
The Safety and Environmental Side
No chemical introduction is complete without a nod to safety. It's tempting to call something “green” and leave it at that, but nothing’s ever that simple. According to data from the European Chemicals Agency, N-Octylpyridinium Tetrafluoroborate poses less risk than classic solvents, yet handling it with gloves and goggles makes sense. Its tetrafluoroborate part contains boron and fluorine, which means proper disposal matters—especially at scale.
Green chemistry isn’t only about replacing one risky chemical with something new. It's about designing substances that do the job and keep health and the planet in mind. Growing up near a creek that kids used to avoid every spring because of runoff made me appreciate projects that treat safe disposal and environmental impact as more than afterthoughts.
Looking Toward Progress
Plenty of challenges remain. Large-scale synthesis and cost put the brakes on widespread adoption in commercial settings. Researchers are testing ways to recycle or recover this salt after use, which could lower the barrier for sustainable chemistry in industry. Investment from public and private sectors will decide how fast new technologies built from such compounds hit the mainstream.
For anyone peeking behind the curtain, N-Octylpyridinium Tetrafluoroborate signals a move toward smarter chemistry. Its rise offers a glimpse at how choices in the lab ripple out to manufacturing, the environment, and everyday life. As science keeps asking more from its building blocks, the push for safety, performance, and responsibility walks right beside every new discovery.
The Backbone of a Chemical's Identity
N-Octylpyridinium tetrafluoroborate tends to grab the attention of chemists and researchers for good reason. With growing interests in ionic liquids, specialty surfactants, and even electrochemistry, knowing the details sets the stage for solid understanding. It isn’t just another compound on a shelf; its makeup tells a story of charged bonds and practical uses.
Molecular Formula: C13H22BF4N
Digging into its structure, the molecule combines a pyridinium ring (a six-membered aromatic ring with one nitrogen atom) and an octyl chain, bolstered with a tetrafluoroborate counterion. The full formula is C13H22BF4N. Each piece fits for a reason— the pyridinium head brings polarity, the octyl tail handles the hydrophobic side, and the tetrafluoroborate holds up its end as a stabilizing anion.
Molecular Weight: 295.13 g/mol
That formula comes out to a molecular weight of 295.13 grams per mole. You feel the importance of knowing this number each time you prep a reaction or calibrate a solution. Miss the mark on the weight and the whole experiment slides away from reproducibility. In labs, small errors in molecular weight mean wasted resources or, worse, faulty data.
Real-World Relevance: Experience and Application
Back in an undergraduate lab, I remember running into trouble with calculation errors. Set-ups using salts like N-Octylpyridinium tetrafluoroborate rely on getting the basic facts straight. Getting hands-on, you see right away that theory means little without these nuts and bolts lined up.
This ionic compound plays out in electrochemistry, helping shuttle ions in systems where stable, non-volatile electrolytes matter. Wastewater researchers poke at it for its surface-active traits, trying to mop up pollutants in tricky conditions. In all these cases, skipping a basic like the formula or molecular weight turns trust in results into guesswork.
Facts, Reliability, and Making Data Matter
Facts don’t flourish in a vacuum. As researchers, we look for peer-reviewed sources and dig into literature. ChemSpider, PubChem, and reliable suppliers list the C13H22BF4N formula and a molecular weight of 295.13 g/mol, matching up with the broader chemical data pool. When information lines up from multiple trusted places, you start to build confidence in your methodology.
Better Data, Better Outcomes
Stepping into a research lab, I saw the impact of that accuracy first-hand. Colleagues leaned on these small chemical facts, knowing that the right data shapes efficient experiment design. Mistakes trickle down to wasted reagents, rework, or inconclusive outcomes. For students and seasoned researchers, sticking to well-supported facts pays off in credible results and smoother collaboration.
Looking Ahead: Solutions for Chemical Knowledge
Accuracy starts early. Chemistry professors need to give students exposure to primary data sources and reinforce the importance of double-checking molecular information. Lab managers can set protocols that prioritize reliable databases. For the broader field, online education and digital resources can bridge information gaps— strong basics mean stronger innovations down the line.
N-Octylpyridinium tetrafluoroborate isn’t just a list of numbers. It’s proof that sound science grows from verified, concrete details— the kind every researcher should reach for, every time.
Understanding the Risks in the Lab
Handling chemicals demands a good eye for detail. N-Octylpyridinium Tetrafluoroborate — a mouthful, for sure — brings its own set of worries. This salt, used in synthesis or as an ionic liquid, isn’t something anyone can just toss on a shelf and forget about. My first brush with this chemical came after cleaning up a poorly labeled secondary container. Everything looked fine, but lingering doubts about stability hung over the workspace.
Keeping It Away from Trouble
A few basics always keep me out of harm’s way. First off, exposure to moisture spells bad news for this salt. Humidity might break down the chemical or cause unwanted reactions. It sits best in a well-sealed, airtight container. I check seals every week—just to stay ahead of leaks. Anyone leaving the lid loose can set off a chain of events that stains lab benches and wastes good material.
The spot for storage matters. I stick it on a dedicated shelf, away from acids and bases. This way, accidental mix-ups never start fires or give off noxious fumes. I’ve read about strange color changes and bubbling when someone stored it near strong acids. That story convinced me: keep incompatible substances far apart and label shelves clearly. Real accidents don’t show up in the textbooks until someone messes up.
Protecting the Chemical and the People
Direct sunlight doesn’t belong anywhere near this salt. Fluctuating light and heat degrade sensitive chemicals long before the label expires. The stockroom I trust most uses amber bottles for anything even a little bit light-sensitive. Stashing containers in a cool, dry place keeps everything stable. My rule: if you’d feel comfortable storing chocolate there, it works for this salt too.
I don’t just trust the container either. Desiccant packs fit right into storage boxes. They grab stray moisture and send signals when they stop working. The steadiest hands can still drop a bottle, so all my containers wear clear hazard labels and emergency instructions. Lectures about PPE fade pretty quickly, but simple reminders on storage bins stick with people.
Preventing Contamination and Spills
Cross-contamination once ruined an entire batch of our catalyst prep. Chemical dust drifts through the air or clings to gloves, and it doesn’t care whose experiment it spoils. I avoid scooping or pouring over open containers and swap out scoops after each use. Spills start small and get out of hand fast, so absorbent mats go beneath high-use areas. We’ve dodged plenty of headaches by tracking inventory and posting checklists around high-risk materials. If a spill does happen, I always want to know where the spill kit is before I need it.
Making Storage Part of the Culture
Storing N-Octylpyridinium Tetrafluoroborate isn't just a chore on the checklist. It’s about keeping everyone safe and saving money in the long run. Good habits pass from one person to the next, and a tidy chemical shelf tells visitors someone cares. The more structure around chemical safety, the less anyone has to scramble during an emergency. Storage isn’t just a technical step; it’s a culture that separates careful labs from careless ones.
Understanding the Compound
N-Octylpyridinium Tetrafluoroborate falls under the family of ionic liquids, grabbing the attention of chemists for more than two decades. Its structure puts together a pyridine ring, an octyl group, and a tetrafluoroborate anion. In the lab, it pops up in organic syntheses and sometimes plays a part in green chemistry research. This isn't a household cleaning product or a snack additive — it's a specialty chemical designed for science.
Weighing the Evidence: Safety and Health Concerns
Most folks outside the lab won’t cross paths with N-Octylpyridinium Tetrafluoroborate. For researchers working with it, the question of safety rides up quickly. Looking at its relatives, such as other pyridinium salts, signals the need for caution. Studies on similar ionic liquids show toxicity toward aquatic organisms and the potential to disrupt cell membranes. Pyridinium compounds, if inhaled, can irritate the respiratory tract. Ingestion isn’t a good plan—animal studies point to liver and kidney stress.
Real data on N-Octylpyridinium Tetrafluoroborate itself is thin. European Chemicals Agency lists it with warnings: it produces serious eye irritation and may cause skin irritation. Handle it in a glove box or fume hood, and never let your guard down. Spills on skin or inhaling dust may lead to acute symptoms. Long-term effects remain a grey area because robust chronic toxicity data has yet to show up in peer-reviewed journals or public safety databases.
Environmental Impact: Not Just a Lab Problem
Think about what flows down the drain. Some ionic liquids don’t break down easily, raising questions about buildup in waterways. Experiments done on freshwater organisms reveal that certain pyridinium salts can stall growth or cause reproductive issues. No one has dropped a truckload of N-Octylpyridinium Tetrafluoroborate into a river to check, but common sense and past examples from similar compounds suggest its environmental footprint should be taken seriously. Ignoring that risk would be a bad mistake.
Handling and Solutions
Walking into a lab means setting yourself up with protection: gloves, goggles, and a solid lab coat. Standard operating procedures teach us to treat unknowns with care until proven safe. Disposal often lands this compound in the hazardous waste category. Facilities equipped with proper incineration or chemical treatment offer one route. Pouring leftovers down the sink isn't just bad manners—it's against environmental regulations.
Companies aiming to use these types of chemicals in industry should push for more testing. Researchers can step up efforts to create better reporting on both acute and chronic effects. Sharing findings through open channels and international chemical safety networks moves science forward and helps protect the people who make innovation happen.
Personal Perspective
With a few years working alongside plenty of sharp chemists, I have seen what happens when new specialty chemicals show up with unknown risk profiles. Labs run better and accidents drop when no shortcuts are taken on safety, even for small-batch experiments. My own approach rests on the rule—never trust a novel molecule without treating it like a hazard. Over time, regulation tends to catch up, but that lag can be costly if people cut corners.
Staying smart starts with education, ongoing research, and strict lab routines. Better to overestimate risk and sleep easy than let a new compound’s unknowns catch you off guard.
What Matters about Purity
Purity isn’t just a buzzword for chemists—it’s a hard limit on what you can expect when you open a new bottle of N-Octylpyridinium Tetrafluoroborate. Too many seasoned researchers have spent hours chasing down impurities or trying to trace contamination, and it rarely ends well. In my own research work with ionic liquids, a reliable supply often means looking for at least 98% purity or even higher. It’s that margin between “usable science” and unpredictable results.
When a supplier claims a compound has 98% or 99% purity, they’re telling you this product contains almost no side products or residual moisture. A stray percent or two might not sound like much, but for experiments using this compound as a phase transfer catalyst, impurity sometimes spells disaster. Even one extra percent can mess with reaction yields or throw off reproducibility.
Global suppliers often calibrate their grades to match lab needs. In my own case, I look for a certificate of analysis—because the best suppliers share data on NMR, IR, and elemental analysis, so I don’t have to wonder what’s in the jar. If you’re after analytical work or working with pharmaceuticals, never settle for a vague “reagent grade.” Most respected manufacturers offer this chemical at 98%, 99%, and some even push towards 99.9% for truly demanding applications.
Packaging Sizes—Real-World Options
Most projects don’t need a 25-kilogram drum of N-Octylpyridinium Tetrafluoroborate. Practically, most labs will see bottles in the 1 gram to 100 gram range. A common order for high-value ionic liquids could be 5 grams, especially at the research stage. That’s not just about convenience—it’s about shelf life and minimizing waste. I’ve often seen chemical suppliers offer 1 gram, 5 gram, 10 gram, 25 gram, and 100 gram bottles as their primary options.
University labs sometimes ask for custom sizes, mostly because their budgets or research protocols demand it. Suppliers usually charge more for smaller bottles, but overspending on a large bottle leads to excess that slowly degrades—with tetrafluoroborate anions, moisture can be a real headache. Messy storage or long-term exposure can trigger nasty decomposition, so smaller bottles keep things stable.
Larger packaging, such as 500 grams or even several kilograms, targets commercial scale-up or repeated use in process chemistry. From what I’ve seen at industry shows and distributors like Sigma-Aldrich, Alfa Aesar, and TCI, they’ll only offer bigger containers if there’s demand; sometimes this means placing a special order, which bumps up lead times.
What Stands in the Way—and the Workarounds
Price volatility hits hardest at high purity and specialty sizes. Good-quality N-Octylpyridinium Tetrafluoroborate isn’t sitting on every shelf, because handling and synthesis can be tricky. Some suppliers cut corners if you let price drive the conversation, so sticking with trusted sources pays off—less worry about batch-to-batch differences, better support if a contamination issue ever comes up.
Supply chains took a hit lately for some specialty chemicals, with long shipping times straining research schedules. Reliable suppliers post their current inventory levels and lead times transparently, and smart labs keep extra on hand to bridge any gaps. Sometimes collaborating with a nearby group or pooling orders saves money and ensures steady access.
Solid relationships with suppliers help. Request batch records, ask about recent purity trends, and never be shy about sending a compound for independent analysis. That attention to detail saves projects from costly repetition or, worse, flawed data.