N-Octylpyridinium Trifluoromethanesulfonate: An In-Depth Commentary
Historical Development
Chemists and industry professionals have always pushed for better and more reliable ionic compounds. N-Octylpyridinium Trifluoromethanesulfonate stepped into the scene as a result of these ongoing efforts. Its history connects to the work on ionic liquids and functional salts that became a hot topic at the end of the twentieth century. Early research focused on the creation of stable pyridinium-based salts, which researchers realized could shift entire methods in organic synthesis and advanced material science. Organic chemists kept digging, and through trial, error, and experimentation, laboratories developed more stable variants. Compound purification improved as the need to meet higher purity standards in electronics and pharmaceuticals grew. After decades of refining synthetic routes and purification methods, the trifluoromethanesulfonate counterion emerged as a favorite, marking a turning point for performance and utility. The journey of this compound, from a laboratory curiosity to an industrial mainstay, mirrors so many stories in the fine chemicals world, where small breakthroughs eventually change the way people build, clean, and innovate.
Product Overview
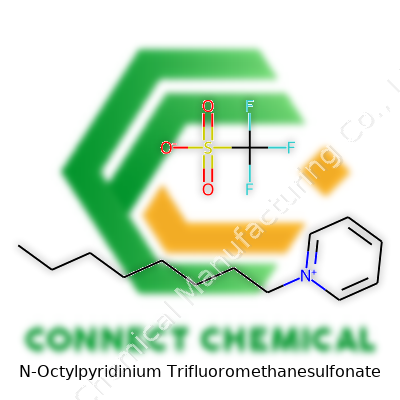
N-Octylpyridinium Trifluoromethanesulfonate takes the structure of a pyridinium ring linked to an octyl side chain and is balanced by the robust triflate anion. This molecule doesn’t enter the market under just one label; suppliers may call it Pyr1C8 Triflate, N-Octylpyridinium Triflate, or even by more technical product codes. At face value, it appears as a white to off-white powder, blending the surface activity of pyridinium cations with the robust chemical stability of the triflate group. Laboratories source this salt for its rigid purity standards, with manufacturers touting 98% or better material. That attention to detail draws in sectors from advanced synthesis to materials research. What draws eyes in the chemical catalog is its versatility, not just a set of numbers.
Physical & Chemical Properties
The physical profile of N-Octylpyridinium Trifluoromethanesulfonate tells a story of balance between organic and inorganic influences. Its long octyl chain gives it a slick, waxy feel at room temperature; this helps with handling and stirring in solvents like methanol, ethanol, or even polar aprotic liquids like acetonitrile. Chemically, the compound resists decomposition under mild thermal and oxidative stress. The trifluoromethanesulfonate anion lends it the kind of stability not found in halide-based analogues — fluorine atoms make this compound resilient against hydrolysis and electrophilic attack. The compound’s ionic structure influences solubility, which proves helpful in formulations where mixed-phase reactions happen, such as in certain biphasic catalysis systems. Despite being a salt, its solubility profile stands apart from simple table salt or common alkali salts, easing dispersion in a range of organic solvent systems.
Technical Specifications & Labeling
Commercial shipments and laboratory samples of N-Octylpyridinium Trifluoromethanesulfonate carry detailed technical spec sheets. These usually describe molecular weight near 395.44 g/mol, melting points ranging from 70°C to 95°C, and trace impurity levels. Chromatograms and spectroscopic results appear in the certificate of analysis, helping researchers fit the material straight into validated workflows. Labels list shelf life, recommended storage conditions (cool, dry, away from direct sunlight), and often hazard information relevant for laboratory and workplace safety teams. Regulatory identifiers like CAS number and REACH status feature prominently to smooth international supply. For niche applications involving pharmaceuticals or electronics, providers offer certificate packages that affirm compliance with pharmaceutical GMP or electronic-grade purity limits — this isn’t the sort of off-the-shelf chemical that lacks a paper trail.
Preparation Method
Laboratories synthesize N-Octylpyridinium Trifluoromethanesulfonate through a two-stage process. The standard method starts with octylpyridine, which reacts with an alkylating agent — usually methyl trifluoromethanesulfonate or a triflic anhydride — in a controlled, inert atmosphere. The basic pyridine nitrogen undergoes clean alkylation, typically under mild heating and vigorous stirring. After the primary reaction step, purification follows. Laboratories use recrystallization from solvents like acetonitrile or ether, or they might opt for chromatography when higher purity is required. The deliberate stepwise approach ensures minimal production of unwanted byproducts. Yield improvement never stops, and companies often keep proprietary tweaks close to their chests, as small purification upgrades can have big impacts on downstream usability. Waste streams are typically managed through separation units and solvent recovery processes, meeting both environmental and economic requirements.
Chemical Reactions & Modifications
The strength of the pyridinium core and the stability of the trifluoromethanesulfonate anion allow N-Octylpyridinium Trifluoromethanesulfonate to stand up to a range of synthetic conditions. In organic chemistry, it operates as a phase-transfer catalyst, ferrying reactive ions between aqueous and organic layers. The octyl side chain can be lengthened or shortened in syntheses to tailor solubility or hydrophobicity for specific industrial uses. Researchers have leveraged its cationic nature for ion-exchange experiments, and its inclusion in ionic liquid formulary gives rise to tunable solvents for challenging industrial extractions. Derivatives pop up through functionalization of the pyridine ring, affording opportunities to add reactive sites or linkers for polymer or dendrimer synthesis. The non-coordinating triflate anion sidesteps unwanted side-reactions, allowing the cation to display full chemical reactivity without interference.
Synonyms & Product Names
In the commercial catalog or academic literature, names for this molecule can vary widely. Aside from the full IUPAC name and the straightforward ‘N-Octylpyridinium Trifluoromethanesulfonate,’ buyers may encounter synonyms such as Pyr1C8·OTf, Octylpyridinium Triflate, or simply as a derivative of OTf salts. Chemical suppliers sometimes use coded designations tied to cataloging or proprietary batch systems, which simplifies reordering but can complicate literature reviews. This web of terminology reflects a common headache in specialty chemical circles, where the same compound might be listed under different aliases by independent vendors or researchers.
Safety & Operational Standards
Handling N-Octylpyridinium Trifluoromethanesulfonate follows chemical industry safety benchmarks. The compound, while not highly toxic, deserves routine respect in the lab. Gloves and safety goggles remain basic requirements during handling, especially in open bench settings or when preparing large samples. Operators avoid generating fine dust and keep the compound away from sources of moisture to prevent premature decomposition or clumping. SDS data highlights possible irritation upon skin or eye contact and suggests quick rinsing with water after exposure. Proper ventilation cuts down on accidental inhalation. For larger operations, standard chemical fire procedures stay in play, given the organic side chain content. Most facilities store the compound in a cool, dry cabinet — humidity and excessive temperature swings undermine long-term stability. Waste protocols for this compound rely on solvent-compatible cleanup, with final disposal following hazardous organic waste guidelines common at university and corporate labs.
Application Area
The chemical community relies on N-Octylpyridinium Trifluoromethanesulfonate for a host of reasons. In organic synthesis, it acts as a phase-transfer salt, easing reactions that combine polar and nonpolar reagents in a single flask. Catalysis research draws on its stable ionic profile — it brings better selectivity and reaction rates to transformations involving nucleophiles or organometallic intermediates. Electrochemical engineering finds a friend in this compound, using it as a conducting salt in prototype batteries, supercapacitors, or ion-exchange membranes. In analytical chemistry, it stabilizes ionic environments in chromatography or mass spectrometry applications. Biochemistry groups experiment with it for antimicrobial screening: the cationic surfactant properties break down bacterial cell walls, suggesting possible paths toward new disinfectants or bioactive agents. Some specialty polymers incorporate this salt to lend durable, charge-carrying features, expanding its reach across the field of advanced materials research.
Research & Development
R&D teams keep stacking use cases for N-Octylpyridinium Trifluoromethanesulfonate. From my years spent in academic and industry settings, seeing a single well-characterized salt like this one pop up in catalytic screening, antimicrobial exploration, and energy storage design speaks volumes about its flexibility. Ongoing research hunts for higher-conductivity derivatives for electrochemical devices or tailors the octyl chain to balance solubility and bioactivity. Environmental chemists dig into greener synthesis or recycling schemes to cut the environmental cost of specialty ion salt manufacture. Documentation from sponsored research and peer-reviewed articles helps build trust for newcomers, who want evidence that the product works as advertised. This cycle, where new application discoveries feed back into process development, stays healthy thanks to open data, robust technical support, and willingness to publish both successes and setbacks.
Toxicity Research
Toxicological tests show N-Octylpyridinium Trifluoromethanesulfonate doesn’t pack the acute punch that some cationic surfactants bring, but caution stays front and center. Animal model data shows low LD50 values at high doses, with gastrointestinal and dermal absorption as the main routes. Chronic toxicity remains understudied, especially regarding breakdown products in the environment. Toxicity panels from third-party labs provide comparative insight, placing this compound in the medium-risk bracket for industrial use — not as dangerous as many legacy solvents, but not a candidate for low-regulation bioactive applications. Proper ventilation, PPE, and strict waste control should block most realistic routes of exposure. Serious work remains for researchers who want to know the long-term ecological impacts of large-scale use, especially if downstream release into waterways or soil becomes a concern. Demand for this data keeps rising, especially as regulations tighten on perfluoroalkyl substances (PFAS) and related chemistries.
Future Prospects
The outlook for N-Octylpyridinium Trifluoromethanesulfonate hinges on the same drivers shaping the rest of specialty chemicals: sustainability, performance, and regulatory clarity. Labs and factories want higher efficiency with a lighter environmental touch. Makers of the compound have started offering greener production options, using bio-based feedstocks or closed-loop purification cycles to cut emissions and reduce byproduct loads. In energy storage, this salt and its family members play a key part in unlocking safe and high-output ionic conductors for batteries that do not rely on lithium alone. Medical researchers explore modifications that turn this basic structure into new antimicrobials or drug delivery aids. Over the next decade, expect tighter regulation and more exhaustive toxicity assessments, but also faster-paced innovation, especially as new sectors — from biotech to sustainable plastics — pick up this compound for uses beyond what early adopters imagined. Collaboration between academia, regulatory groups, and industry will stay crucial as the story of N-Octylpyridinium Trifluoromethanesulfonate keeps unfolding.
A Closer Look at Its Role in Chemistry
N-Octylpyridinium Trifluoromethanesulfonate might look like a mouthful, but the main story boils down to its work as an ionic liquid in research labs. I’ve spent enough time chatting with chemists and reading through published papers to see a pattern: researchers keep reaching for this compound because of its ability to act as a high-performing phase transfer catalyst. This quality comes from its unique ionic structure, where the pyridinium core brings stability to reactions and the octyl group boosts its solubility across a range of organic solvents.
What Makes It Valuable in Synthesis?
In organic synthesis, certain reactions struggle because reactants land in the wrong layer—one in water, the other in oil, so to speak. N-Octylpyridinium Trifluoromethanesulfonate steps in and bridges the gap, helping reactive partners meet where they're most active. Take nucleophilic substitution: this compound makes life easier for chemists who want to speed up or steer reactions. Its ionic nature lets it pick up charges and shuttle them between the oil and water worlds. When you need to build molecules with precision—think pharmaceuticals or specialty chemicals—missing this tool slows everyone down.
Supporting Innovation Beyond the Lab
Away from synthetic benches, N-Octylpyridinium Trifluoromethanesulfonate pops up in electrochemistry. Its ionic liquid qualities show up in advanced batteries and capacitors, especially where high conductivity and thermal stability matter. The trifluoromethanesulfonate anion (triflate for short) resists breaking down at high voltages and temperatures, which is a plus for electronics makers hunting for reliability.
This task isn’t just about making things work today. As energy storage tech slowly crawls toward more reliable and greener solutions, researchers keep experimenting with ionic liquids like this one. From what I’ve seen, this path will continue, especially as industries push for batteries that last longer and handle bigger loads without catching fire or leaking chemicals.
Addressing Safety and Environmental Concerns
No chemical compound walks into the spotlight without questions. Concerns about toxicity and environmental impact always follow fancy new synthetic tools. The literature points out that pyridinium-based salts, especially those carrying long alkyl chains like the octyl group, can threaten aquatic life if dumped without treatment. This triggers the call for better protocols in handling, disposal, and, where possible, recycling. Research teams I know monitor their waste streams and invest in scrubbing technologies, but smaller outfits sometimes fall behind.
Looking Ahead: Smarter Applications and Replacements
Chemists want to have their cake and eat it—high performance without headaches. That’s why, alongside using N-Octylpyridinium Trifluoromethanesulfonate for its top features, innovators keep eyeing greener ionic liquids or tighter regulatory checks. Substituting shorter-chain or biodegradable salts, supporting closed-loop systems, and tightening control on laboratory effluents stand out as ways forward. Facing today’s scrutiny, researchers balance the drive for progress with a personal and professional stake in safety and responsibility.
Experience tells me: breakthroughs in the lab often ride the shoulders of compounds like these, but the story doesn’t end with reaction yields. Every new use brings a trail of choices about safety, environmental impact, and practical handling. That’s the real ground level of progress—figuring out how to innovate without leaving a mess behind.
Chemical Identity: Getting Down to Brass Tacks
N-Octylpyridinium trifluoromethanesulfonate steps onto the stage with the chemical formula C14H24F3NO3S. Its molecular weight clocks in at 359.41 g/mol. These numbers might only seem handy for plugging into calculations, but they drive far more than math—accuracy in these details has ripple effects through chemistry research and practical work.
Real-World Roots for the Numbers
Bumping into unfamiliar chemicals in the lab can feel like a trip through a language with no dictionary. Years spent at the bench taught me early: mislabeling or guessing a formula can lead research astray and cost weeks of work, especially with specialty salts that end up as the backbone of ionic liquids or surfactants. N-Octylpyridinium trifluoromethanesulfonate often finds a home in organic synthesis as a phase-transfer catalyst and also appears in antimicrobial studies. Without the right formula, even a small error can spark a chain reaction of wasted effort and bad data.
Why Exact Chemical Formulas and Weights Matter
Many projects in applied chemistry rely on measured substance amounts. Solution concentrations, stoichiometry in reactions, and regulatory reporting all track back to that molecular weight. It’s not a detail that sits idle in a textbook. When I worked on formulating surfactant blends, a one-gram calculation error, born of a forgotten fluorine, left an entire batch off-spec. Scrapping thousands of dollars’ worth of material never leaves your memory. These experiences highlight just how crucial it is to have the right numbers in hand from trusted resources or published literature.
Trust, Transparency, and Sourcing Information
Scientists today need to trust their sources. Chemical structures shouldn’t come as a surprise package, especially not for compounds with a long tail of syllables. Databases like PubChem and European Chemicals Agency entries give up-to-date, verifiable data, and sticking with peer-reviewed sources keeps errors out of circulation. Looking up the chemical formula from a reputable supplier or cited publication, double-checking, and storing the information in lab notebooks can spare hours of headaches later.
Improving Access and Reducing Errors
One simple solution—open-access digital databases—already helps researchers worldwide cross-check molecular formulas, CAS numbers, and weights. In some academic labs, I’ve watched new students keep a running spreadsheet handy, checked and signed off by their advisor. Honest mistakes still happen, but more eyes on every data point help catch them before they go into a publication or production run.
Looking Forward
With complex compounds like N-Octylpyridinium trifluoromethanesulfonate, transparency and double-checking basics lay the groundwork for good science. Lab notebooks, accurate references, and peer review keep the wheels turning smoothly. Being deliberate about data builds a chain of reliability, stretching from a simple calculation on paper all the way to safe, successful use out in the field or on production lines.
Keeping Chemicals Safe: Lessons from the Lab
Anyone who’s spent time in a working laboratory knows the hassle that pops up from poor chemical storage. One overlooked bottle, set too close to a heat source or left on a windowsill, can throw off an entire experiment or, worse, spark safety issues. N-Octylpyridinium Trifluoromethanesulfonate has become more common in research labs, thanks to its use in specialty synthesis and as a catalyst. Handling this compound, like any modern organofluorine salt, means not cutting corners when it comes to storage.
What Matters with Organofluorine Salts
Chemical integrity starts with temperature. N-Octylpyridinium Trifluoromethanesulfonate should stay out of both the freezer and direct heat. Room temperature, defined as around 20-25°C, works best. Swinging between hot and cold doesn’t just cause clumping or sticking; it can degrade the salt, making it less effective during experiments. I’ve seen talented researchers waste whole weeks because of tiny cracks in their storage routines.
This compound doesn’t like dampness. Moist air can sneak in once a cap loosens, leading to clumps and sometimes even slow decay. Standard practice calls for storing it in a tightly sealed container – the kind with a screw cap and a proper gasket. Some labs go with desiccators or cabinets with silica gel packs, especially in the summer or in humid climates. This reduces the chances of water sneaking in.
Light: The Sneaky Degrader
Few people pay much attention to light until a sample changes color or behavior. Ultraviolet rays and even the bright halogen bulbs found in busy labs can alter chemicals that otherwise look stable. Sticking N-Octylpyridinium Trifluoromethanesulfonate in an amber glass bottle helps, but closing it away in a drawer or opaque cabinet works even better. Makeshift fixes like wrapping bottles in foil go a long way. Over years, I’ve watched careful storage routine extend shelf lives, save money, and reduce hazardous waste.
Labeling: A Basic Step that Saves Futures
Sloppy labels create confusion, especially when handling organofluorine compounds. Every container should wear a clear, legible sticker with its full name, date received, and lot number. Many labs also record storage conditions on these labels, short and sweet, so someone new can spot if a bottle spent time outside the recommended temperature or humidity range.
Personal Responsibility and Team Training
Safety officers can only do so much if the daily users forget the rules. Regular training on chemical storage, including walk-throughs and visual reminders right by the storage shelves, keeps everyone on the same page. I remember a colleague who made a habit of showing new team members exactly where moisture-sensitive compounds live. That simple routine prevented at least one costly accident every year.
Better Storage, Lower Risk
Proper storage of salts like N-Octylpyridinium Trifluoromethanesulfonate saves on replacement orders and avoids slow disasters such as contaminated reactions or gradual bottle leaks. Investing in well-sealed containers, climate control, and honest labeling makes a real difference. The little habits we build around chemical safety don’t just keep experiments on track – they keep people safe over the long run. That’s something no training manual should ever gloss over.
Getting to Know the Chemical
N-Octylpyridinium Trifluoromethanesulfonate sounds like a mouthful, but in most labs, complicated names often stand for useful tools. This substance isn’t something you’d find in a grocery store, but in research, it can serve as a phase transfer catalyst or pop up in ionic liquid chemistry. Not everyone stumbles upon this powder unless their work leans deep into organic synthesis or specialty applications.
Checking the Hazards: What Experts Say
Reading into the safety sheets, one thing stands out: this chemical doesn’t play around with human health. Symptoms like skin or eye irritation are possible, as nearly all pyridinium salts can cause issues if they get on your hands or, worse, your face. Users also report respiratory discomfort if inhaled, thanks to the triflate part of the salt, which tends to be sneaky because fine powders become airborne easily. Unlike some of the nastier substances out there—cyanides, for example—this one doesn’t rate as acutely toxic, but assuming it’s harmless just because it’s new on the shelf leads to trouble.
No Corner-Cutting with Handling
No matter what research project feels urgent, gloves and goggles stand between a chemist and slow-burn health issues. Open bottles inside a fume hood, because triflate salts can become a cloud before anyone realizes what’s happening. The same applies to weighing or transferring this material. Gowning up in a lab coat isn’t overkill; it prevents contamination, both for your skin and any sensitive reactions nearby.
Reaching for the right gloves can mean the difference between a quick day at the bench and weeks spent wondering what “sensitization” means. Nitrile gloves do the job. Cotton won’t stop the powder from sneaking in, and latex can sometimes degrade, especially after a spill or splash.
More Than Just Direct Contact
Worried coworkers talk about dust control for a reason. Pyridinium salts end up everywhere if users get careless. Without sealed containers, contamination spreads to doorknobs, notebooks, even coffee cups. I’ve seen the mess that comes from not cleaning up well, and it’s just trouble—think unexplained headaches or coughs, which people never connect back to the last experiment until it’s too late.
Don’t just leave the containers sitting open or toss spills down the drain. It sounds obvious, but stories of ruined waste streams and clogged pipes repeat every semester in teaching labs. Trifluoromethanesulfonate parts don’t just dissolve and vanish; they can linger, react, or build up—none of which makes janitors happy.
Smart Storage and Safe Disposal
Dry, tightly sealed bottles in a cool spot keep this material from breaking down or absorbing trash from the air. Walk-in chemical closets with real ventilation handle compounds like this best. If your building only owns a basement closet, double up on containers.
Disposal should involve a hazardous waste program familiar with ionic compounds. Dumping any amount into common garbage poses an environmental hazard, especially if triflate anions slip into water systems. Local regulations help keep these rules clear, but in my years working around specialty salts, the labs that follow their own rules endanger more than just their staff—they risk fines and, more importantly, unexpected trips to the hospital.
A Matter of Training and Attention
No shortcut works. Lab managers and research staff need to remind themselves and their teams about updated safety protocols and keep Safety Data Sheets handy, not buried in a drawer. Respecting chemicals, even obscure ones, means normalizing good habits every day. The right gear, steady attention, and healthy skepticism of all white powders on the bench shape a safer place to do science.
Digging Into the Numbers: Purity Matters
Experience in the lab shows that purity isn’t just a number on a label—it truly changes what you can achieve. N-Octylpyridinium Trifluoromethanesulfonate usually lands around 98% or higher in terms of purity from reputable chemical suppliers. That couple of percentage points might look small, but anyone who’s spent time troubleshooting experiments knows contaminants have a way of taking over. Especially with materials used in organic synthesis, catalysis, or research applications, unchecked impurities often skew results or block reactions.
Researchers count on that high level of purity for reproducibility. I’ve seen results go sideways in spectroscopy tests when a compound turned out to have a significant percentage of residual solvent. Even 1-2% unknowns can lead to confusing results, especially in sensitive reactions. Trusted suppliers back up their claims with certificates of analysis. Skipping that paperwork usually leads to wasted effort and budget, as I’ve learned from past mishaps.
Packaging Sizes: Matching Need to Quantity
In my own work sourcing specialty chemicals, I rarely want a single large drum of something this specific. N-Octylpyridinium Trifluoromethanesulfonate typically comes in small bottles, starting at a gram and running up to 25- or even 100-gram containers. For pilot scale or early research, most people stick with the smaller side—nobody wants to tie up funds in a shelf of obscure salts. Sometimes, vendors offer custom packaging, but that comes with longer lead times and minimum order requirements.
I’ve spoken with colleagues who have requested bulk lots, and they’ll tell you the custom route only makes sense for a group working on a specialized process, such as developing custom electrolytes for batteries or surfactants for formulation research. For academic and industrial teams handling novel compounds, just a gram or five delivers enough material to run a series of controlled tests or exploratory syntheses.
In my experience, 5- to 25-gram bottles hit the best balance. They’re easy to store in chemical cabinets, offer enough material for repeated experiments, and help keep dangerous waste or spoilage to a minimum. Opening a fresh bottle for each project cuts down on cross-contamination—a lesson learned from old stock going bad or absorbing moisture.
Why These Details Deserve Attention
I’ve had projects stall because the spec sheet hid critical information. When suppliers aren’t clear about what percentage purity you’re really getting, troubleshooting gets rough. Contaminant signals show up in spectra and LC-MS runs, and loose troubleshooting can blow apart timelines or kill a publication’s chances. Sourcing the right amount, packaged in a way that fits lab workflow, saves real trouble—not just for researchers, but also for QA teams keeping an eye on process reliability.
A little due diligence on purity levels and package sizes pays off. Trusted suppliers will show batch analysis data; avoid anything lacking that. If a team expects to scale up or repeat a synthesis, asking for 10 grams rather than five often results in greater consistency between batches. I’ve found that clear communication with vendors, attention to storage conditions, and up-front budgeting for the right quantities can all help avoid supply headaches down the line.
For many labs, N-Octylpyridinium Trifluoromethanesulfonate means something complex and mission-critical—a compound that won’t tolerate cut corners. If the lab is building on top of earlier results, purity and consistent supply let you focus on discovery rather than damage control.