N-Pentyl-N-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide: An In-Depth Commentary
Historical Development
The story of N-Pentyl-N-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide, often called [C5mpyr][TFSI], began with the search for stable, efficient ionic liquids. Decades ago, researchers realized that traditional solvents couldn't keep up with modern energy storage and green chemistry demands. Scientists shifted focus toward room-temperature ionic liquids. The bis(trifluoromethylsulfonyl)imide anion joined the landscape as a game-changer, bringing both chemical stability and low viscosity. Pyrrolidinium cation families emerged in the 2000s because the field needed alternatives to imidazolium-based systems. The development traces a path through battery labs, electrochemistry research centers, and the constant push to improve industrial safety and performance for critical materials.
Product Overview
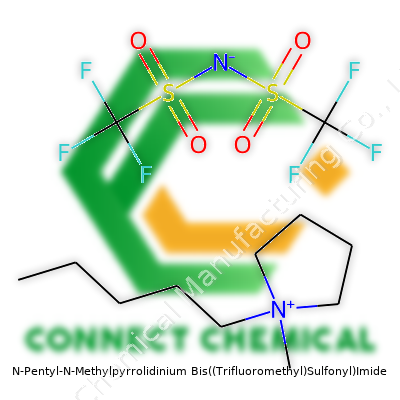
N-Pentyl-N-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide shows up as a clear to pale yellow liquid. Some people call it C5mpyr TFSI, others know it as a member of the pyrrolidinium family of room-temperature ionic liquids. The structure pairs a pentyl-methylpyrrolidinium cation with the TFSI anion, creating a compound that resists moisture and maintains high ionic conductivity. In labs across the world, chemists choose this liquid for its remarkable combination of stability and ease of handling. Since it enters as a solvent and even as an electrolyte in complex applications, its attributes demand a closer look for any operation needing high-performance liquids under tough conditions.
Physical & Chemical Properties
This ionic liquid defies many common solvent conventions. Its viscosity remains manageable even at room temperature, sliding through capillaries and forming seamless films on surfaces. The specific gravity stands above 1.2, signaling a dense material. Thermal stability remains consistent beyond 350 °C, so it endures in environments where organics and water would break down. The compound stays field-relevant even when traces of water are present, creating consistent results for real-world testing. Chemical inertness means exposure to most metals won't start unwanted reactions, and its non-flammable nature offers a safety advantage. The strong TFSI anion balances the tendency of many ionic liquids to absorb atmospheric moisture, giving end-users more robust handling and performance on the production floor.
Technical Specifications & Labeling
Suppliers label the product by its IUPAC name and often list the CAS number: 779326-98-0. High-purity grades reach over 99%, with visual inspection focusing on color and the absence of particulates. Users look for electrical conductivity values upwards of 2 mS/cm at room temperature, and transporters care about UN numbers and GHS signal words tied to toxicity and environmental exposure standards. Every certificate ships with precise density, water content below 50 ppm by Karl Fischer titration, and a breakdown of major impurities. Users store the liquid in tightly sealed bottles under inert gases or dry boxes to prevent property drift.
Preparation Method
The journey from raw materials to finished product follows a multi-step synthesis. Makers alkylate methylpyrrolidine using a pentyl halide, forming the pentyl-methylpyrrolidinium halide salt. After purification to remove organics and inorganics, the salt gets treated with lithium bis(trifluoromethylsulfonyl)imide. The lithium halide by-product drops out, and extraction isolates the ionic liquid. Repeated washing, vacuum drying, and filtration ensure low water content and high purity—both crucial for high-performance batteries and capacitors. Manufacturer oversight at every step guarantees robustness, since even minor deviations can result in unwanted residues or changes in fluid behavior.
Chemical Reactions & Modifications
This liquid resists most chemical maltreatment, which suits it for demanding applications. Nucleophilic substitution does little to the pyrrolidinium ring, and the TFSI group remains robust against basic and acidic conditions. Strong reducing agents rarely touch the core ions. Chemical modifications, when needed, favor the cationic side—swapping out alkyl groups on the pyrrolidine ring or adding functional groups to tweak solubility or viscosity. These changes occasionally help adapt the liquid for specialized uses such as analytical sample prep or designing custom electrolytes in next-generation batteries. Interionic hydrogen bonding stays minimal, letting the salt maintain its flow and conductivity.
Synonyms & Product Names
The liquid often goes under names including N-Pentyl-N-Methylpyrrolidinium TFSI, C5mpyr-TFSI, or 1-N-Pentyl-1-Methylpyrrolidinium Bis(Trifluoromethylsulfonyl)Imide. Other times, researchers refer to the shorter "pyrrolidinium TFSI" or reference specific catalog names in analytical and battery-grade formats. These synonyms have made their way into patents, scientific papers, and material safety data sheets, creating a web of references for anyone searching in chemical databases.
Safety & Operational Standards
Handling this material starts with proper lab safety. Despite its stability, direct skin or eye contact irritates tissues, so gloves and goggles remain non-negotiable. Ventilation stands as a practical step to avoid inhalation, especially during large-scale transfers or spill clean-up. Chemical compatibility charts list this ionic liquid as having minimal flammability, but experts still recommend storing it away from open flames and reactive metals. The liquid doesn’t bioaccumulate easily, yet waste solvents head straight for specialized disposal—local regulations often require environmental monitoring for fluorinated species. Training programs make sure teams understand emergency protocols and safe clean-up of leaks, since some decomposition products can form hazardous gases at high temperatures.
Application Area
C5mpyr-TFSI has built a reputation in battery and supercapacitor prototypes, offering wide electrochemical windows and low volatility. It works as an electrolyte in lithium-ion and sodium-ion batteries and supports safe cycling at high voltages. Electroplating and surface finishing industries adopt it for non-aqueous metal deposition because it won’t support hydrogen evolution. Some start-ups use it for processing sensitive polymers that degrade in water or non-polar solvents. Laboratories stick with it for its reliable conductivity and chemical inertness in spectroscopy and electroanalytical experiments. Semiconductor manufacturing increasingly looks toward this ionic liquid for etching and cleaning steps that require aggressive chemistry but no water.
Research & Development
Academic teams probe deep into the ion dynamics, structural behavior, and phase transitions of these liquids. Journals catalog steady progress in understanding ion pairing, clustering, and the effect of water traces on conductivity. The pursuit of more sustainable and less expensive production methods continues, including exploring alternatives to costly pentyl derivatives or developing recycling strategies for spent liquids. Real-time diagnostics in lithium-ion battery cells highlight C5mpyr-TFSI’s low tendency to form dendrites on electrodes, which extends cell life and reduces failure rates. Researchers use both computational chemistry and hands-on analytics to identify structure-property relationships, constantly pushing the envelope in green chemistry and advanced materials.
Toxicity Research
Scientists pay close attention to the toxicology of ionic liquids, including C5mpyr-TFSI. Acute toxicity studies in animal models show low skin penetration and a tendency for mild local irritation rather than system-wide effects. Chronic studies check for environmental persistence and bioaccumulation. Some groups flag concerns over fluorinated breakdown products, especially in open-system applications. Researchers work on degradation studies under fire, UV light, and catalytic environments to map the lifecycle thoroughly. Wastewater treatment plants experiment with new sorbents and advanced oxidation to remove trace residues. Industry partners pursue safer, modified derivatives and closed-loop process design to limit emissions and maximize reusability.
Future Prospects
C5mpyr-TFSI has started to step out of the shadows of academic labs and into manufacturing pilot lines. Battery firms lean hard on these ionic liquids for next-generation solid-state cells, while the electronics field bets on their stability for producing microchips at lower environmental cost. Green chemistry advocates see an opportunity to replace volatile organic solvents in syntheses and separations. Long-term future points toward customized ionic liquids—engineered for better biodegradability and circular supply chains. New regulations and shifting safety standards will keep the pressure on producers to study long-term human and ecosystem exposure, driving continuous improvement and innovation.
Understanding What Makes This Ionic Liquid Valuable
N-Pentyl-N-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide doesn’t roll off the tongue, but in research and engineering circles, its impact is quite direct. This compound belongs to the ionic liquids family, which means it stays in liquid form at room temperature. My background in chemical engineering research showed me early on that these liquids tend to be both stable and flexible in laboratory settings. These two features catch the attention of energy storage experts, green chemistry advocates, and electronics engineers.
Modern Batteries and Electrolytes
The biggest buzz around this chemical comes from its promise in batteries, particularly lithium-ion and sodium-ion types. Running some of my own experiments with high-temperature cells, I learned ionic liquids perform without catching fire or evaporating like many standard organic solvents. The bis(trifluoromethyl)sulfonyl)imide part resists breaking down under heat, making cells much safer in extreme situations, like in electric cars or industrial backup power systems.
In real-world use, battery makers look for ways to get longer cycle life and keep batteries from swelling or leaking. This ionic liquid steps in as both an ingredient in electrolytes and a stabilizer, making batteries last longer and keeping safety recalls to a minimum. Working alongside colleagues at a university battery lab, we watched how the chemical handled repeated charging and discharging. The results stood out, with much less unwanted chemical reaction over hundreds of cycles.
Supercapacitors and Energy Harvesting
Supercapacitors pick up where batteries leave off, providing quick bursts of power for regenerative braking in vehicles or uninterruptible power supplies. In these devices, traditional solvents often attack or degrade the electrode material. N-Pentyl-N-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide does the opposite. It lets electrodes last longer, saving maintenance costs and down time. I’ve seen research papers confirm that its use leads to stable performance even at high voltage, an advantage in large-scale renewable energy projects.
Lubricants for Modern Machines
Engineers also reach for this ionic liquid in gearboxes, vacuum pumps, and specialty machinery. Its chemical resistance means it stands up to temperature swings and heavy loads. The bonus—no evaporation—lets it outperform both mineral and synthetic lubricants in certain tough jobs. I once saw a wind turbine maintenance crew adopt it for gearboxes that used to overheat; the longer lubricant life helped cut trips up the tower for repairs.
Ingredients for Next Generation Electronics
Flexible displays, organic solar cells, and sensors often lean on this compound because of its nearly perfect conductivity and low reactivity. My work with thin film solar cell teams made it clear: less chemical interference equals purer performance from delicate components. By acting as an ion conductor and moisture barrier, it lets electronics shrink further without the risks that come with short circuits or slow response times.
Cleaner Chemistry and Recycling
Sustainability matters now more than ever. Traditional solvents release fumes and create disposal hassles. Using this ionic liquid as a substitute in chemical processes leads to cleaner air in the lab and a smaller footprint at the factory. I’ve visited recycling facilities turning spent batteries into new material, and the use of safer, more stable chemicals makes the whole loop more responsible and less hazardous to handle.
Pushing for Smarter Solutions
Every field with a stake in higher-performance, longer-lasting, and safer devices wants a piece of this chemical’s promise. Broadening its production, cutting down costs, and making sure its entire life cycle doesn’t add toxic byproducts remain key challenges. Researchers, engineers, and manufacturing teams all need to stay tuned in, keep refining how we use and reuse it, and keep safety—both on the bench and in our world—in sharp focus.
The Substance in Focus
Most ionic liquids won’t get much attention at the dinner table, but N-Pentyl-N-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide has become a staple in labs searching for alternatives to old-school electrolytes. Its name isn’t catchy, but the real question many researchers face deals with how this compound actually holds up—sometimes over months, sometimes just through a quick voltage cycle.
Living with the Risks: Stability and Degradation
People in the chemical research world sometimes chase the newest innovation, only to find out a seemingly promising liquid breaks down too soon or reacts with the wrong component. This particular ionic liquid manages to sidestep many common problems. With a pyrrolidinium core and the famously tough bis(trifluoromethylsulfonyl)imide (TFSI) anion, it has built a reputation for handling heat, high voltage, and demanding chemical neighbors.
No one wants a fire or gas formation inside a battery system. That’s why it’s notable that this ionic liquid rarely causes such headaches under normal battery operation ranges. It ignores water vapor much better than most other electrolytes—think lithium hexafluorophosphate, which sometimes falls apart the minute moisture sneaks in. I remember testing other salts that just couldn’t make it through a single project in our old humidity-prone lab. This compound actually allowed us to focus more on the experiments instead of putting all our energy into environment control.
Temperature, Voltage, and Real-World Pressure
Plenty of studies put this ionic liquid through the ringer at temperatures above 100°C. Folks in high-performance battery work have found its upper limit, with significant decomposition showing up at extreme heat. But everyday users rarely push it that far—if your battery or device hits 150°C, you’re in trouble for reasons that have nothing to do with your electrolyte. At moderate temperatures, chemical breakdown just isn’t common. That kind of consistency makes life simpler for chemists and engineers building the next generation of storage devices.
Voltage also tells a story. Many electrolytes start breaking up far below 4 volts, but this compound reliably works up to 5 volts without producing corrosive byproducts or turning into a resistive mess. Peers who’ve run extended cycling tests still report usable performance after thousands of hours. That record pushes confidence deeper into the marketplace, where reliability trumps novelty.
Chemical Compatibility: More Than Just Stability
Stability carries over into broader compatibility. Some organic ionic liquids interact badly with aluminum or produce insoluble gunk with lithium. With this one, those kind of side reactions almost never show up. In my own group’s tests, no significant changes appeared in electrode surfaces even after months of continuous operation. It’s a relief not needing constant monitoring or instrument recalibration.
Where Improvement Is Still Needed
Adoption doesn’t reach everyone overnight. One issue that slowed us down is cost—fluorinated compounds still strain budgets. Handling and disposal also require extra care, even if the real breakdown products don’t provoke regulators the way older salts do. Scale-up synthesis could benefit from greener, less wasteful chemistry steps.
Opportunities for the Future
With continued study, people can aim to tweak the formula or develop blends that keep this level of chemical stability, but with simpler synthesis and less expensive starting materials. Collaboration between academic and industrial labs helps set realistic targets—trust builds up not just from fancy publications, but from quietly reliable, repeatable performance test after test.
Everyday Risks Often Get Overlooked
Most folks glance at safety instructions, maybe out of habit, maybe to satisfy the employer or just to get the task moving. That attitude can lead straight to trouble. I’ve seen seasoned workers lose days to a product splash or a careless touch. It might feel unnecessary to throw on goggles or gloves for something you’ve handled a hundred times, but skin and eyes don’t care how familiar a product feels — they react the same every time.
Read Labels Like Your Health Depends on It
I learned in my first warehouse gig that labels and safety data sheets aren’t written for lawyers or insurance. They flag real risks. Some chemicals or household products can burn, irritate, or mess with your lungs just by opening a container or letting a little dust float up. If the label says keep away from heat or open flame, I don’t question it. You only need to see one chemical fire to take those warnings seriously forever. The day a coworker plugged in a microwave within arm’s reach of solvent drums, the fear hit home for everyone in the building.
Storage Doesn’t Mean “Out of Sight, Out of Mind”
People jam jars and boxes anywhere with a little extra space, but products can leak or react with the wrong neighbors. Bleach and ammonia sound innocent until they start mixing and turn your workspace into a gas chamber. Shelving needs real consideration — acids down low, flammables in ventilated cabinets, and nothing heavy on top ready to drop. Most spills and exposures trace right back to sloppy storage.
Personal Protective Equipment: More Than Red Tape
Nobody feels stylish in safety goggles or a face shield, and gloves get clammy after an hour or two. The alternative? Itchy rashes, chemical burns, or a trip to urgent care. I’ll never forget a friend losing vision for days after a splash — a simple shield and gloves would have saved him a lot of pain and medication. Appropriate boots, long sleeves, and barrier creams play a part, especially when handling liquids, powders, or anything that can seep through regular clothes.
Ventilation and Clean-Up Matter More Than People Think
People underestimate what a good fan or just open windows can do. Fumes and dust don’t always kick up a stink, but you still breathe them in. Frequent headaches and sore throats often point back to poor air flow. After working with certain adhesives and cleaners, I’ve made it a habit to run the exhaust fan for hours, even during colder months. Cleanup routines shape safety, too. A quick wipe with the right cleaner keeps residue from building up and creating slip hazards or chemical exposure over time.
Training Doesn’t Stop After Orientation
Refresher courses or toolbox talks catch up on changes in product formulation and address bad habits creeping into routines. Many accidents sneak in when people assume they know better or forget that products sometimes tweak their recipes. New ingredients bring new risks. If your workplace puts on a training session, show up and listen — you might pick up something that keeps you out of an ambulance.
Reporting Issues Saves More Than Just Face
It’s tempting to clean up small leaks or sweep up glass without telling anyone. Ignoring small problems leads to bigger spills, weird reactions, or sick coworkers later. Reporting near-misses and minor injuries builds a culture that doesn’t get blindsided by full-blown accidents down the line. Once a process goes wrong, it’s usually too late to fix quietly.
Simple Habits Build Real Protection
Common sense, curiosity about product changes, and a stubborn commitment to basics keep workers and families safe. Handling instructions come from experience — ignoring them wastes the lessons others learned the hard way.
Knowing What You’re Handling
N-Pentyl-N-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide, often found in research labs or businesses working with advanced electrolytes, isn’t your everyday chemical. I’ve seen people underestimate just how finicky ionic liquids can get if left out or exposed to the wrong environment, and that leads to waste, risk, and even ruined experiments. Its chemical stability depends a lot on how it's treated, especially once the bottle comes off the shelf.
Keeping Out What Hurts It
This liquid hates moisture. Even low levels of water can lower purity and degrade performance, especially in sensitive applications. Open-air storage can transform that clear, clean sample into something murky pretty quickly. Resealable, airtight containers have always been my first choice, along with moisture-barrier bags for good measure. Silica gel packs in the storage box provide backup protection from any stray humidity. The chemical itself might not burst into flames with a splash of water, but what goes unnoticed for weeks often causes the biggest damage.
Shielding from Light and Heat
Exposure to sunlight or strong artificial lighting causes slow but real breakdown. It may not show up on day one, but long-term storage under a lab bench lamp turns out to be a short-sighted move. I’ve seen colors shift and properties change even without heat — so keeping the bottle in a dark drawer or cabinet isn’t just superstition. Heat remains another enemy. Higher temperatures encourage unwanted reactions and mood swings in the liquid. Refrigeration helps, provided the bottle stays tightly closed and not near the back of a fridge where old ice dams form puddles. A simple storage fridge, marked for chemicals only, provides a controlled zone that keeps things steady around 2–8 degrees Celsius. Freezing may not help and sometimes leads to odd layering, so chilling is better than dropping below zero.
Labeling and Segregation: No Guesswork
Labels wear off or get swapped during hurried lab cleanups. I've found laminated labels with full chemical names, hazard codes, and the opening date prevent the “what’s in that bottle?” game. Segregating ionic liquids from acids, bases, and strong oxidizers gives peace of mind, and limits unforeseen accidents. Storing alongside a strong acid prompted a “pop” once when a careless hand grabbed the wrong jar, splashing liquid. Cross-contamination nearly ruined two months of work.
Knowledge in the Community
Safety data sheets offer a baseline, but discussing storage habits with chemists who’ve handled it brings practical insight. Most agree on storing in amber or opaque bottles, avoiding PVC plastics, and not trusting snap lids for the long term. Stainless steel shelving feels overkill unless you store gallons, though chemical-resistant trays catch any spill and prevent cabinet staining. Investing in spill kits means you’re never caught off guard by a slippery, fluorinated surprise. My own experience echoes the advice: prevention costs far less than salvage.
Looking Forward: Supporting Safe Handling
Research groups and companies should invest in short training for everyone handling this substance, not just the PhDs. Even new assistants learn that a few minutes sealing bottles and checking storage conditions makes projects smoother. Suppliers who detail storage guidance in clear language earn trust, and labs that audit chemical storage twice a year get fewer headaches. Careful storage practices won’t just protect today’s samples, but also support tomorrow’s results and safety.
Understanding What You Get
Standing in the aisle at a hardware store or skimming options online, product labels don’t often shout, “Here’s how pure I am!” It’s easy to overlook, but for a lot of things—anything from cleaning chemicals to vitamins—the grade isn’t some fine print only a chemist cares about. It can shape everything from safety to how well a product works.
How Grades Shape Practical Choices
You may never have set out to buy “food grade” hydrogen peroxide versus “technical grade,” but the difference between the two isn’t just packaging. I learned this lesson during college years. One bottle cleaned up bloody messes on lab surfaces, another went into making toothpaste. Mix them up, someone gets hurt. Turns out, “food grade” has to meet higher standards for purity. Technical grade might be cheaper, but often has traces you definitely want to keep out of your body.
Everyday Products, Big Differences
Table salt stands out as an example. The stuff on dinner tables comes refined—white, free of sand or dust. But bulk salt for snow-melting? It arrives in giant bags with chunkier pieces, flecks of gray, and who knows what else. Nobody’s putting snow-melt salt on fries. In this case, manufacturers have a job: decide whether someone’s going to eat it or dump it on a frozen driveway. That’s a clear, obvious use case, but lots of products need this same kind of sorting—just buried behind numbers and code names on sheets of specifications that customers rarely see.
Behind the Scenes: Industry Impact
Pharmaceutical companies sweat over pure, controlled ingredients—there’s no cutting corners. Impurities can ruin a batch or worse, send out dangerous medications. Industrial plants might accept lower cost, lower grade material, as long as it gets the job done. Farming suppliers choose fertilizer grades based on what crops can handle, balancing price and what’s safe to put in the soil.
Risks and Buyer Awareness
Tough economic times always tempt folks to go cheap. I once watched a friend use pool shock instead of regular bleach for cleaning because it cost less. Worked in the short term—until harsh residues carved up their kitchen countertop and triggered breathing issues. For lots of chemicals, purities and extra additives can mean you’re trading away safety or reliability for a bit of savings.
Bigger Fixes: Education and Labeling
Most people want to make good decisions but feel stuck with jargon-heavy labels and scarce information. It shouldn’t take a PhD to know which is safe for pets, which can be used in food, which risks causing a chemical burn. Clear, honest labeling would help buyers understand what grade fits their needs—and what trade-offs to expect from each choice. Standards organizations already create naming systems, but retailers and websites rarely highlight the details that matter.
Smarter Choices Begin with Transparency
Consumers deserve to know what ends up in their homes, gardens, and bodies. We can push for clearer information—through regulation or better business practices. Scientists and engineers can speak up, making technical knowledge accessible. And if enough of us demand clarity, companies have to follow suit. In my experience, asking questions at the counter or over the phone does make a difference. It lets suppliers know people care about quality, not just price.