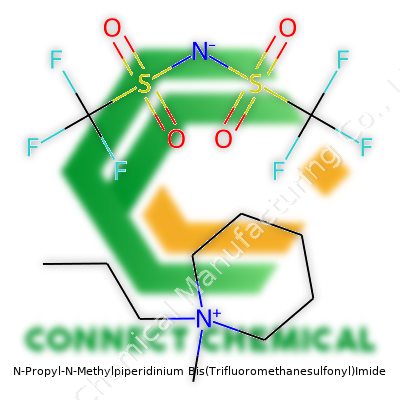
N-Propyl-N-Methylpiperidinium Bis(Trifluoromethanesulfonyl)Imide: Commentary on History, Uses, Properties, and Future
Historical Development
Scientists exploring new frontiers in chemistry regularly search for compounds that can stretch the limits of traditional chemical formulations. N-Propyl-N-Methylpiperidinium Bis(Trifluoromethanesulfonyl)Imide entered the scene as research into room-temperature ionic liquids picked up steam over the last few decades. Early studies into ionic conductors for batteries often included more familiar compounds, but the growing need for thermal and electrochemical stability led researchers in the late twentieth century to piperidinium salts paired with the bis(trifluoromethanesulfonyl)imide anion. People working in laboratories recognized the limitations of previous generations of ionic liquids, often constrained by high viscosity or troublesome reactivity, and pivoted to exploring quaternary ammonium-based ionic liquids as promising alternatives. A few decades ago, access to these specialty chemicals ran up against barriers of expensive synthesis and limited purity. But consistent effort by research teams in Japan, Germany, and the United States led to reproducible preparation of this compound at higher yields, making it accessible for a broader suite of experiments targeting next-generation materials.
Product Overview
The appeal of N-Propyl-N-Methylpiperidinium Bis(Trifluoromethanesulfonyl)Imide, often shortened as PMP-TFSI within scientific circles, comes from a balance between structure and performance. It joins a class of ionic liquids valued for both chemical resilience and high ionic conductivity. The structure replaces a straightforward methyl group with a clever piperidinium backbone, then adds propyl to tune amphiphilic properties. Pairing it with the TFSI anion means a chemical stable against oxidation, plus a backbone that is bulky enough to discourage lattice formation, so it stays fluid over a wide range of conditions. Researchers select this compound to sidestep the volatility or corrosion hazards tied to older salts like perchlorates. It finds a place in the toolbox for those looking to push electrochemical boundaries, whether building new battery prototypes or testing materials for supercapacitors.
Physical and Chemical Properties
Unlike simple salts or common solvents, PMP-TFSI jumps out due to its low melting point and remarkable thermal stability. Colorless or pale liquids are routine, with viscosity heavily influenced by the length and branching of the alkyl chains. The compound won’t boil at room temperature; it persists in liquid form under a full range of climates seen in most labs. Many recognize its almost negligible vapor pressure as both a safety and environmental asset, lowering risks of inhalation and reducing losses to the atmosphere. On the chemical side, PMP-TFSI handles strong bases and acids with relative ease, showing little inclination to break down under regular laboratory conditions. In moisture-rich environments, it largely resists hydrolysis compared to imidazolium-based alternatives. Solubility varies—the compound blends into many polar solvents but holds together in the face of non-polar hydrocarbons, so it resists phase separation when used in certain electrolytes. Specific gravity often falls just above one, so spills sit and are easy to spot and clean safely.
Technical Specifications & Labeling
Manufacturers follow strict purity standards because trace impurities upset the delicate balance in electrochemical cells, especially where device safety matters. Analytical data sheets often list PMP-TFSI at 98% or better purity, with water levels kept well below 100 ppm and total acid or base number less than 1 meq/kg. Product containers generally come in dark glass or moisture-proof canisters, labeled with the correct UN number and signal words according to Globally Harmonized System standards. To support downstream research, lot-specific certificates of analysis detail not just major composition but also trace metals, which can poison reactions in sensitive applications. Every reputable vendor tracks lot numbers to match traceability demands seen in pharmaceutical and electronic sectors, responding to the push for quality assurance and accident prevention.
Preparation Method
Synthesis begins with N-methylpiperidine. Alkylation with n-propyl bromide or chloride forms the N-propyl-N-methylpiperidinium halide as a quaternary ammonium salt. Anion exchange takes place by stirring this intermediate with lithium or sodium bis(trifluoromethanesulfonyl)imide in water or acetone. Mixing the two leads to precipitation of sodium or lithium halide with the desired ionic liquid forming as a distinct liquid or separable phase. Purification steps include repeated washings with water to clear out all residual inorganic salts, followed by careful drying over molecular sieve to meet strict moisture specs. Sometimes this compound passes through an extra filtration step to remove microscopic particles that could disrupt sensitive electrochemical systems. Scale-up requires attention to both keeping out moisture and minimizing exposure to light, since trace photolysis could shape unwanted byproducts.
Chemical Reactions and Modifications
Once synthesized, the backbone resists unwanted modification, which is a positive trait for repeat use in demanding setups like batteries. Some teams experiment with alkyl chain length, tweaking the propyl group or swapping methyl for longer chains to adjust viscosity, hydrophobicity, or solubility in various organic phases. Protonation of the piperidinium ring rarely occurs under standard conditions, so the chemical remains stable in acidic solutions. Pairing with different anions opens up additional chemical space—some labs switch out TFSI for other large, non-coordinating anions to compare differences in ionic conductivity or thermal limits. The core cation shows high resilience against redox cycling, resisting breakdown in fuel cells or during repeated charge-discharge cycles in batteries.
Synonyms and Product Names
Chemists and suppliers don’t always speak with one tongue, making it easy to miss a product during literature searches if names differ. N-Propyl-N-Methylpiperidinium Bis(Trifluoromethanesulfonyl)Imide also appears as 1-Methyl-1-Propylpiperidinium Bis(trifluoromethanesulfonyl)imide or PMP-TFSI. Sometimes catalogs shorten it to [PMP][TFSI] in research-focused products. Trademarks or catalog entries list it as a specific grade or formulation, distinguishing battery-grade from generic laboratory batches based on trace impurity and water content. Some patent literature prefers the term “ionic liquid electrolyte containing PMP-TFSI,” so researchers looking to cross-reference patents with academic articles need to keep these variations in mind.
Safety and Operational Standards
On a safety level, this compound rates better than many legacy salts but still warrants respect. Direct contact leads to irritation, and repeated skin exposure builds up over time. Laboratory safety data sheets require gloves, goggles, and local exhaust ventilation to keep users clear of accidental splashes or inhaled vapors created during high-temperature processing. Unlike old halide-based electrolytes, this compound does not pose a major explosion risk unless heated near decomposition temperatures above 300°C. Disposal follows local hazardous waste guidelines—its high fluorine content makes it persistent in the environment, so neutralization and incineration at permitted facilities are the norm. Accidents get documented as part of chemical hygiene programs, and ongoing education targets younger lab members as older chemists retire. Mistakes in handling concentrated PMP-TFSI can lead to expensive cleanup and lost time, making operational discipline critical in both industrial and university settings.
Application Area
Researchers working in advanced batteries, supercapacitors, and electrochromic devices find PMP-TFSI indispensable. Its blend of low volatility and high oxidative stability turns it into a go-to electrolyte for lithium-ion and sodium-ion cells, especially where performance at high temperature matters. Some high-profile projects use it in dye-sensitized solar cells to boost cycle life under extreme sunlight. A handful of articles document its use as a plasticizer in polymer electrolytes, where its unique structure gives flexibility to otherwise brittle materials. Industrial coatings pick up its resilience, using it as an additive to boost resistance to chemical attack or modify surface properties of membranes. During my own experience working with prototype energy storage, using PMP-TFSI made it possible to cycle cells at higher voltages—giving us weeks, not just days, of steady performance.
Research and Development
Chemical research doesn’t advance in a straight line; setbacks and surprises push teams to look for new formulations all the time. Over the last five years, growth in interest around next-gen batteries rekindled attention on ionic liquids like PMP-TFSI. Papers presented at electrochemical society meetings feature real cell data, comparing life cycles of nearly identical cells with and without this compound. Beyond energy storage, current research investigates how mixtures with other ionic liquids tune phase diagrams, possibly lowering cost and improving transport. At the University of Tokyo and Stanford, several research groups measure the impact of systematic impurities, finding that even 100 ppm water steps down conductivity by more than ten percent—so the drive for purer, more consistent batches continues. Private sector labs studying solid-state battery chemistries roll out new blends on the back of this compound, acknowledging patents that credit its stability over earlier contenders. Collaborative work crosses oceans, with public data from European consortia broadening access for institutions with less in-house synthesis power.
Toxicity Research
Toxicity research stands out as a necessary companion to performance testing. Early animal and cell assays showed this compound does not readily bioaccumulate, yet chronic low-level exposure in closed environments raised eyebrows. Some evidence points to the TFSI anion as the main ecological worry—persistent in water and showing modest toxicity to aquatic life over time. Disposable gloves and local exhaust reduce lab worker risk, but plant-scale production still needs ongoing environmental monitoring. Researchers publishing on workplace safety recommend updated exposure guidelines for all quaternary ammonium-based ionic liquids, not just PMP-TFSI, since downstream effects from tiny spills build up over decades. A few studies out of Europe placed the NOAEL (No Observed Adverse Effect Level) conservatively, meaning long-term use in mass consumer goods should proceed only with clear safety data in hand, not just performance measurements.
Future Prospects
Looking ahead, the fate of PMP-TFSI depends on how well industry and academia balance cost, performance, and steady improvement in safe handling. Some see it as one step in a long transition to safer, more robust ionic liquids for energy technology. Synthetic routes will likely shift toward greener reactants and solvents as regulatory pressure against fluorinated byproducts mounts. The next generation of lithium-ion and sodium-ion batteries almost certainly expects to see PMP-TFSI in one or another form, especially in applications where flammability and volatility force designers to rethink old formulas. The chemical’s adaptability unlocks whole fields beyond energy storage, possibly serving in pilot-scale catalysis or as a solvent for delicate chemical separations. Scalability, recycling, and improved toxicological data all sit near the top of the to-do list for enterprises betting on steady commercial markets over speculative one-off sales. In my years observing trends at major research conferences, those compounds that walk the line between innovation and reliability end up shaping industries well beyond their original intent, and PMP-TFSI stands as a strong contender in that arena.
Vital Ingredient in Safer, Next-Generation Batteries
N-Propyl-N-Methylpiperidinium Bis(Trifluoromethanesulfonyl)Imide, often called a mouthful of an ionic liquid, does some real work behind the scenes in energy storage. I remember reading about fires caused by unsafe battery electrolytes; they sparked a rush for safer alternatives. Li-ion batteries need stable mediums for ion movement, and this compound stands out. It offers remarkable thermal stability and doesn’t catch fire like some old-school solvents. This matters for electric vehicles on highways and for the phones we hold by our beds. Lithium-ion cells rely on it to enhance safety margins, make fast charging possible, and extend lifespan.
Step-Change for Capacitance in Supercapacitors
I once helped a friend tear down an old backup power supply. We found that conventional capacitors relied on liquid electrolytes that would either dry out or break down. N-Propyl-N-Methylpiperidinium Bis(Trifluoromethanesulfonyl)Imide doesn’t degrade under extreme voltages or high cycles, so supercapacitors using it reach higher voltages and survive years of charging and draining. This pushes performance in quick charge-discharge devices for regenerative braking on trains and grid-scale energy balancing.
Creating a Greener Lab Bench for Electrochemistry
During my grad school stint, our lab kept searching for solvents that wouldn’t fume or corrode equipment. This ionic liquid remains stable under air and moisture. It opens up possibilities in synthesizing sensitive compounds, reducing reliance on hazardous chemicals. Colleagues working in industrial electroplating appreciate its reliability during metal deposition, especially for specialty electronics and catalyst work.
Efficient and Robust Heat Transfer
Think about data centers pumping out serious heat. Cooling liquids with low vapor pressure and no flammability concerns sound like a dream. This ionic compound helps cool electronics where leaks can’t be allowed because it won’t evaporate and conducts heat effectively. I’ve read about engineers testing it for heat-transfer fluids in harsh environments, where standard coolants break down under load.
Potential for Safer, High-Performance MEMS Devices
Micro-electromechanical systems (MEMS) pack sensors into cars, smartphones, and industrial robots. They often need stable, non-volatile fluids for actuation and lubrication. Device miniaturization puts pressure on all materials used, so fluids that won’t evaporate, corrode, or degrade become life-savers for engineers. This compound meets the mark in long-term reliability tests that others can’t touch.
Real-World Challenges and Solutions
Cost can trip up adoption, as the chemistry of making these ionic liquids isn’t cheap. Scaling up production while keeping purity high poses hard questions. Strong research partnerships between academia and industry can help tune synthetic methods, clear regulatory hurdles, and lower prices. Public funding for pilot projects, like the efforts that drove down costs in lithium cell manufacturing, could play a similar role here.
As energy demand grows and electronics shrink, materials like N-Propyl-N-Methylpiperidinium Bis(Trifluoromethanesulfonyl)Imide move out of obscure academic journals and straight into critical tech—always behind the scenes, making things safer, cleaner, and longer-lasting.
Why Chemical Structure Matters
Chemistry shapes the world in ways a lot of people never give much thought. The structure of a chemical compound isn't some abstract puzzle—it's a recipe card for how that substance interacts with everything around it. The chemical structure tells us how atoms arrange and bond, each link forming the backbone for properties like reactivity, toxicity, and usefulness. Once, during a college internship in an environmental lab, I found out just how important this can be. We discovered a contaminant in water using both the formula and the structure, and it impacted how the city treated its drinking supply. Public safety depended on getting the compound right, not just its name.
What the Formula Tells You
The chemical formula acts like a signature—think C6H12O6 for glucose or H2SO4 for sulfuric acid. The letters show which elements you’re dealing with, and the numbers reveal how many atoms of each. If you work with cleaning agents, fertilizers, or even food additives, you need these formulas to know what’s safe and what reacts in dangerous ways. Each formula means something in plain, practical terms; they aren’t just for scientists. Farmers care about ammonium nitrate (NH4NO3) because of its fertilizer and explosive features. Bakers keep sodium bicarbonate (NaHCO3) handy for lighter cakes. In medicine, molecular makeup can be the line between a lifesaver and a health risk.
Beyond the Formula: Real-Life Consequences
Relying only on a chemical name without knowing the precise formula and structure gets people in trouble. Several years ago, a local disaster occurred when industrial workers misread a drum label, mixing hydrochloric acid with bleach—releasing toxic chlorine gas. The cause traced back to misunderstanding both the structure and hazards of what was in those barrels. The reality is, getting a chemical structure and formula right isn’t just knowledge for chemists or pharmacists—it affects anyone who cooks, cleans, or maintains a home or business.
The Human Face of Chemistry Education
Some teachers drill formulas so much, students forget why they matter. But seeing the link between citric acid in lemonade and C6H8O7 opens new eyes. Food safety recalls often tie back to minor differences in structure—think the difference between edible oils and trans fats. In my work with food manufacturers, I saw how tiny tweaks in structure, invisible to the naked eye, made the difference between something nutritious and something that flunked health standards. Chemical structures aren’t just for lab coats; they matter in lunchrooms, garages, and farms.
Solutions That Make a Difference
Everyone deserves clear, trustworthy information about substances they handle daily. Schools can use hands-on kits so every child sees molecules as building blocks, not mystery codes. Fact sheets for workplaces, with visuals for chemical structures and formulas, cut confusion and accidents. Kitchens and home workshops grow safer when families learn to recognize more than just product labels. Tools like smartphone scanning apps can now identify compounds from barcodes or QR codes, showing both the formula and a simple structure diagram. The future looks brighter with plain-language instructions, not just chemistry jargon.
Building Trust, One Molecule at a Time
Responsible reporting and science communication relies on facts, transparency, and practical knowledge. The chemical structure and formula should never seem tucked away in academic journals. Everyday decisions, from pesticide use on crops to food preparation at home, rest on understanding what these structures mean. Accurate chemistry makes daily life safer and turns science from mystery into a useful ally.
Why Storage Practices Matter
N-Propyl-N-Methylpiperidinium Bis(Trifluoromethanesulfonyl)Imide, like many ionic liquids, boosts performance in batteries and electrochemical devices because of its stability and conductivity. This compound brings innovation, but mishandling turns a useful material into a source of occupational hazard. Scientists and technicians want to shield people and environments from unnecessary risk. Years in a research lab taught me that a safe workbench often starts with looking after chemicals nobody else wants to think about.
Containers and Environment
Plastic reacts to all sorts of stuff, so glass bottles with screw caps often hold up stronger for storing this compound. Select an airtight, chemical-resistant container. Anyone who has reached under the counter and touched sticky residue understands leaky containers lead to headaches at best, health problems at worst.
Low humidity and cool temperatures help maintain stability. I’ve watched liquids degrade and form unidentifiable crusts on a hot shelf in summer. High heat speeds up decomposition. High humidity, especially in a cramped back room, lets compounds attract water. Moisture not only ruins purity but also increases the chances of hazardous reactions.
Workplace Organization
A cluttered bench or forgotten shelf in the corner invites trouble. Shelve this compound away from food, acids, and oxidizers. Keep incompatible materials apart based on their reactivity, not only by alphabet. Spill containment trays beneath storage bottles catch drips before they turn into cleanup emergencies.
Label everything. I once spent a long afternoon tracing the source of an acrid smell to an unlabeled beaker. Even if you are the only one using these materials, accurate labeling with date received, chemical name, and appropriate hazard signs avoids confusion later.
Personal Protective Equipment and Ventilation
Simple cotton gloves and standard goggles do not provide full protection here. Nitrile gloves, chemical splash goggles, and a long-sleeve lab coat help block skin contact. Many researchers install a local exhaust hood or use the compound in a fume hood, not only to manage vapors but to add a last line of defense against accidental splashes.
Some ionic liquids can cause irritation, so splashes demand quick action. Clean running water and emergency showers give you a fighting chance to manage accidents. Eye wash stations near the work area should never gather dust.
Building a Safety Culture
Paper safety protocols help, but people follow them only once they see their value. In my experience, emphasizing hands-on training and regular safety meetings makes people more likely to use gloves and goggles without a reminder. Small group demonstrations highlight proper transfer from storage to workbench, and handling spills with confidence, not panic.
Waste disposal stands out as a blind spot in many labs and facilities. Never pour surplus material down the drain. Collect waste in marked solvent-resistant containers and tag for certified chemical waste pickup according to local rules. I’ve seen fines hit organizations hard because of shortcuts with hazardous waste.
Paving Pathways to Safer Labs
Facilities can install real-time air monitoring to alert users if volatile byproducts escape containment. Smart inventory management systems help keep track of batches and expiration dates, reducing old and unstable chemicals piling up unnoticed. Manufacturers should outline clear storage and disposal directions tailored for labs and factory floors.
What’s Behind the Label?
No one grabs a product off the shelf and ponders the purity grade. Most folks just look for the name and maybe the price. Dig a little deeper and the reality jumps out—what’s inside the container can change everything, especially in industries where accuracy decides whether an experiment succeeds, a medicine works, or a piece of hardware lasts.
Real-World Impact from Lab to Living Room
Pharmaceuticals live and die by purity. Something as small as an extra 0.5% of a contaminant can shift the effectiveness of a drug—or turn what’s supposed to help into something dangerous. Chemists I worked with spent more time double-checking certificates of analysis than actually mixing chemicals. That level of caution exists for a reason. Labs order “analytical grade” reagents for reliable results, because lower grades can throw entire testing runs into the trash.
In food production, grades decide both quality and safety. Take salt: table salt, kosher, pharmaceutical, and industrial salt start from the same sodium chloride but carry vastly different requirements for moisture, mineral content, and processing. Nobody would sprinkle de-icing salt on their fries, yet without clear grading, mistakes could slip through. A misstep in grade selection puts the consumer at risk and erodes trust in a brand fast.
Industrial Pressure: Why Grade Isn’t Just About Price
In manufacturing, grade choice impacts more than product cost. Machine shops order specific steel grades because impurities change metal strength and outcome. Builders who ignore grades might save a little upfront, then face catastrophic failures—hard lessons learned with tragic consequences.
In electronics, purity becomes king. A friend of mine works in semiconductor fabrication, where silicon wafers with trace contaminants can kill an entire batch worth millions. Not worth the gamble.
The Drive for Transparency and Safety
Clear communication about available purities gives professionals control and accountability. Hazy labels, loose documentation, or confusing terminology open doors for mistakes. The wider the adoption of reliable grading and transparency, the safer everyone downstream remains.
There’s another issue: international supply chains. Importing chemicals or materials from different countries means facing a maze of standards and naming conventions. Inconsistent grading drags out projects and sparks disagreements. Universal benchmarks—like those built by ASTM, ISO, or USP—help cut confusion.
Solutions: Where Responsibility Begins
Manufacturers carry the biggest share. Labeling products with honest grades, listing contaminants present (even in small amounts), and clearly defining use-cases lets buyers make informed decisions. Regular third-party verification of grades keeps suppliers accountable and keeps bad actors off the market.
End-users have responsibilities too. Professionals should push back on unclear specifications and demand better documentation. Rushing through orders just to cut costs often leads to bigger headaches down the road.
In my experience, a casual phone call with a supplier, asking straightforward questions about purity and use-cases, works better than relying on product sheets alone. Building relationships and raising the bar for transparency—those steps go furthest to protect end-users, patients, and reputations.
Navigating the Maze of Chemical Shipping
Moving hazardous chemicals is no walk in the park. Every shipment carries a set of risks, and missing just one regulation can trigger fines or even accidents on the road. Years ago, I remember standing in a warehouse before a drum shipment. My palms got sweaty reading through the paperwork, wondering if I missed a label or a shipping paper. Those rules can feel endless, but they keep people safe.
Understanding Hazard Classification
Every chemical gets slotted into a specific danger category. The shipping process starts with knowing exactly what the chemical is—flammable liquid, toxic gas, corrosive solid. This matters, since these labels direct everything that follows. The United Nations sets out these hazard classes, and agencies like the US Department of Transportation and European ADR enforce them.
Mislabeling a chemical as non-hazardous, or skipping a red diamond on a box, can land a business in legal hot water. It only takes one leak for a minor mistake to spiral. I’ve seen a missed hazard label halt a shipment for days, costing both time and money, so accuracy from the start pays off.
Packing the Right Way
Shipping chemicals isn’t about throwing drums on a truck and strapping them down. Every container must hold up against bumps, pressure changes, and even drops—sometimes several meters in a warehouse or port. Organizations like the International Maritime Organization spell out what counts as safe packing. If a certain chemical reacts with water, the packaging must keep out every drop of moisture.
The packaging doesn’t just protect products. It guards workers unloading crates and firefighters responding to spills. One summer, I talked to a driver about a leaky barrel incident. He spent hours in an emergency room, not because of a truck crash, but because some folks skipped a seal inspection. That sticks with you.
Paperwork Never Ends
Shipping documents have to spell out every risk, every emergency measure, and every chemical name. Drivers need to show these on demand at checkpoints, border crossings, and ports. Missing documents slow everything down and sometimes lead to rejected goods. Proper shipping papers—the safety data sheet and the emergency contact—act like a passport for each drum or tote of chemicals.
In my own shipping days, I always double-checked those details. Errors create problems miles down the road, both literally and legally. Digital systems help, but you can’t beat the old habit of reading everything twice.
Regulatory Compliance Keeps People Safe
Governments don’t write complex laws just to flex their power. Every step, from labeling to storage, stands on the back of real-life disasters. The Bhopal disaster and Texas City explosion left scars on whole communities, and new regulations came after those tragedies. These rules exist to protect folks working the docks and drivers navigating busy highways.
Following the right guidelines sometimes feels like a drag—costly and time-consuming—but once you see the fallout from skipped steps, the checkerboard of laws starts to make sense.
Improving Chemical Shipping
Factories, trucking firms, and shipping companies should work together to train staff. Regular refreshers on labeling changes and safety data can help avoid costly accidents. Technology helps, but experience in reading a MSDS by hand still pays off. For small businesses, finding a partner with experience in hazardous shipments can take some of the burden off their shoulders.
There’s no shortcut. Chemical shipping needs vigilance and a solid understanding of the rules. The right move? Start by checking the hazard class, confirm container integrity, and always read your paperwork one more time. It can save more than just time; it saves lives.