N-Propyl-N-Methylpiperidinium Hexafluorophosphate: A Critical Chemical for Modern Science and Industry
Historical Development
Chemists started exploring piperidinium salts as far back as the middle of the twentieth century, hunting for safer, more stable, and more flexible ionic compounds. N-Propyl-N-Methylpiperidinium Hexafluorophosphate didn’t appear by accident. Rather, it’s the result of steady work in organic synthesis and electrochemistry, blending established traditions in piperdine chemistry with newer efforts in fluorine-based anions. Through the eighties and nineties, labs in Japan, Germany, and the United States competed to refine quaternary ammonium salts, sharpening synthetic methods and looking for candidates that could take the place of hazardous or less-efficient electrolytes. Researchers gradually narrowed in on the N-propyl-N-methylpiperidinium cation for its manageable synthesis and balance of physical properties, and chemists recognized that pairing this cation with a hexafluorophosphate anion would provide a stable, easily handled salt.
Product Overview
N-Propyl-N-Methylpiperidinium Hexafluorophosphate caught my interest on a deep dive into specialty electrolytes for battery research. This salt exists as a white to off-white powder, odorless and non-hygroscopic, preferred for its broad electrochemical window and non-volatile profile. Modern applications have moved it out of the niche zone and onto the benches of labs tackling ionic liquid research, solid-state electrolyte development, and next-generation separation techniques.
Physical & Chemical Properties
This compound features a melting point around 185–190°C, with a decomposition point that sits noticeably higher, giving it stability for both low- and moderate-temperature operations. It stands out with minimal solubility in non-polar solvents but dissolves efficiently in polar organics, especially acetonitrile and propylene carbonate, pointing to its popularity in electrochemical setups. The cation’s piperidinium ring resists nucleophilic attack, and the hexafluorophosphate anion offers high chemical inertness under normal conditions. The salt sports an ionic character and maintains high conductivity in solution, critical for efficient charge transfer.
Technical Specifications & Labeling
Reputable suppliers provide N-Propyl-N-Methylpiperidinium Hexafluorophosphate with a purity exceeding 99%, accompanied by certificates of analysis listing water content, halide impurities, and trace metals. Labels specify not only the chemical formula (C9H20NPF6) but batch numbers, recommended storage conditions, shelf life, and special hazard symbols if the product’s destined for electrochemical or R&D use. Technicians managing inventories often check these specs before accepting shipments—especially the water content, since moisture saps performance in sensitive cell assemblies.
Preparation Method
The primary route involves alkylation of N-methylpiperidine with n-propyl bromide or n-propyl chloride, yielding the quaternary ammonium halide salt in high yield. After purification, metathesis with potassium hexafluorophosphate in water or acetonitrile swaps the halide for PF6-, leaving behind a clear organic layer, often washed and recrystallized to scrub out leftover halides and reaction byproducts. In small-batch labs, chemists carefully dry solvents and glassware, since even minor levels of water or halide ions throw off subsequent analyses. For upscaling, material engineers watch reactor temperature, monitor agitation speeds, and take care to limit atmospheric exposure, knowing that impurities influence both electrochemical and thermal properties in the finished salt.
Chemical Reactions & Modifications
N-Propyl-N-Methylpiperidinium Hexafluorophosphate plays a role in ion-exchange reactions and as a supporting electrolyte in controlled-potential electrolysis. The compound resists oxidation under mild conditions, which is why it finds its way into high-voltage batteries and supercapacitors. Under forced hydrolysis or with strong Lewis acids, the hexafluorophosphate anion can decompose, producing hydrofluoric acid and phosphoryl fluoride, so storage in dry, inert environments remains critical. Chemists experimenting with cation modifications often swap the propyl group for longer or branched alkyl chains, tuning viscosity and solubility for different applications. Such modifications help unlock new uses in catalysis, chromatography, and advanced material development.
Synonyms & Product Names
The salt appears on technical datasheets and research publications under several names. Chemists shorten it to PMP-PF6 or refer to it as N-propyl-N-methylpiperidinium hexafluorophosphate. Some catalogs list it by its systematic IUPAC moniker: 1-propyl-1-methylpiperidinium hexafluorophosphate, while specialty suppliers attach codes such as “EMP01-00023” or similar, clarifying batch provenance and grading for research or industry use.
Safety & Operational Standards
Working with N-Propyl-N-Methylpiperidinium Hexafluorophosphate in the lab brings back clear memories of constant glove changes and eye shield checks. PF6- based salts call for strict dryness and plenty of ventilation—breathing in breakdown products during decomposition can risk real harm. Material safety data points toward eye, skin, and respiratory irritation. Direct contact isn’t recommended, and emergency protocols demand shower and eyewash access. During disposal, lab operators follow rules for halogenated waste, tracking containers and logging batch IDs to stay on top of compliance with both local and international chemical handling standards. Most producers urge storage at room temperature, away from acids, strong bases, and moisture. Clearly printed expiration dates keep people aware of reactivity changes that creep in over time.
Application Area
Development in lithium-ion battery research drew me to this compound years ago. N-Propyl-N-Methylpiperidinium Hexafluorophosphate offers a winning blend of ionic conductivity and thermal resilience, thriving as a primary or co-electrolyte in high-energy-density battery platforms. Electroplaters use it in specialized metal deposition baths. Analytical chemists adopt PMP-PF6 as an ion-pairing reagent in advanced chromatographic separations, because it tweaks selectivity in stubborn separations where milder agents fall short. At the interface of organic and inorganic chemistry, the salt supports solvent-free syntheses and plays a key role in the advancement of “designer solvents.” Surface scientists study how it assembles at air-liquid and liquid-solid interfaces, uncovering mechanisms that feed into nanomaterial production and fuel cell innovation.
Research & Development
Academic and industrial groups treat N-Propyl-N-Methylpiperidinium Hexafluorophosphate as a springboard for new ionic liquid designs. I’ve seen research explore cation tailoring, not only swapping alkyl groups, but also incorporating functional moieties that transform the salt into a reactive medium or a phase-transfer catalyst. Commercial R&D focuses on enhancing conductivity and electrochemical stability, balancing cost with performance for applications ranging from photovoltaics to advanced antistatic coatings. Government-funded projects often use this salt as a benchmark in electrolyte testbeds, while startups explore integrating the compound into wearable tech, supercapacitors, and flexible solar panels.
Toxicity Research
Toxicological work shows that both short-term exposure and chronic contact can be harmful, especially when hydrolysis liberates HF. Studies using cell cultures and aquatic models report moderate ecotoxicity, so diligent waste handling isn’t just a legal concern—it's a community responsibility. Research on bioaccumulation and long-term human exposure keeps expanding, nudged on by safety-first laboratory culture. I recall detailed training modules highlighting both direct and indirect exposure risks, with plenty of emphasis on keeping sample vials sealed and documenting every transfer or disposal. Regulatory guidance continues to tighten, pushing for improved personal protection, better ventilation, and more refined limits on allowable emissions during manufacture and laboratory use.
Future Prospects
N-Propyl-N-Methylpiperidinium Hexafluorophosphate opens doors for safer, more powerful, and environmentally sensitive electrochemical systems. Researchers look to push stability higher, reach broader voltage ranges, and shrink environmental impact. Next-generation battery technologies lean on this salt and its derivatives, especially as solid-state and high-performance applications move out of the pilot stage. Collaborative projects draw on synthetic chemistry, toxicology, and device engineering to guide modifications. The compound's versatility ensures it will remain a central figure in both established and flowering sectors, with discoveries in electrolyte design, nanostructure assembly, and even green chemistry feeding continual demand for reliable, safer ionic salts.
What’s Hiding Behind the Name?
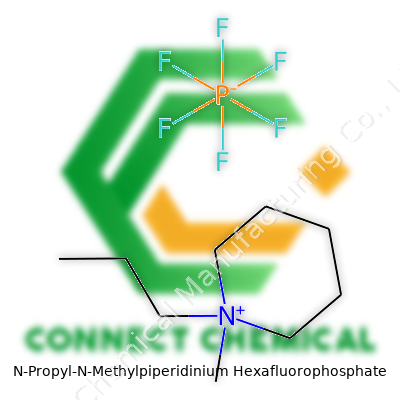
People ask about the structure of N-Propyl-N-Methylpiperidinium hexafluorophosphate, and it sometimes sounds more like a tongue twister than a chemical. Beyond the tough name, this molecule reflects the complicated, ingenious side of chemistry. It brings together a piperidine ring, some clever substitutions, and an anion that’s shaking up labs from batteries to synthesis work.
The Shape of the Molecule
Start in the middle: piperidine has six carbons arranged in a ring, one swapped for a nitrogen atom. It’s like looking at a mini Ferris wheel with the nitrogen as the odd seat. That nitrogen gets both a methyl group (one carbon and three hydrogens, CH3–) and a propyl group (three carbons, C3H7–) sticking out of it. That creates N-propyl-N-methylpiperidinium, a positively charged, chunky molecule.
Imagine the structure: the nitrogen atom sits right in the ring, attached to a string of three carbons (the propyl group) and a stubby single carbon (the methyl). Because it’s attached to four groups, the nitrogen can’t keep its spare electron, so this whole part becomes a cation — it carries a positive charge.
The other part, hexafluorophosphate (PF6-), is the anion, six fluoride atoms sitting around a phosphorus atom like an atomic umbrella. This anion offsets the positive charge from the cation, giving you an electrically neutral salt. Both parts come together, stacking positive and negative charges tightly through simple electrostatic attraction.
Why Does This Structure Matter?
That arrangement isn’t just science fiction. The big, bulky cation resists lining up nicely or forming tight crystals, and the hexafluorophosphate anion won't stick to very much. That combination turns out to matter a lot. Compounds like this show up all over modern chemistry, especially in ionic liquids. These special salts stay liquid at room temperature, dodging the neat, tidy crystal patterns of most typical salts.
Industry folks tinker with the structure to unlock new properties, hoping to find better electrolytes for next-generation batteries. Ionic liquids built from these sorts of cations don’t catch fire easily and handle high voltages, which feels reassuring if you’ve ever had a battery die or overheat in your hand.
Looking at the Bigger Picture
Getting more from ionic compounds means thinking about more than lab experiments. Ionic liquids with this kind of piperidinium skeleton already cut a mark in greener industrial chemistry. People use them for solvent work, electrochemistry, and even as part of safer cleaning processes. They often beat traditional solvents on toxicity and flammability. That offers a win for both lab safety and the push to clean up chemical manufacturing.
Chemists still face big questions: How do these molecules break down in the environment? Can the industry scale up production without hiding waste downstream? The only way to handle that is by testing, transparency, and constant tweaking. So far, published research shows the potential for improved recyclability and less hazardous byproducts, though work on the full lifecycle impact is ongoing. Some labs also push for using less rare ingredients in their synthesis, cutting reliance on finite resources.
Continuing Down the Road
Chemistry doesn’t march forward unless people dig into details like this. Tinkering with the rings and tails, swapping out anions, and tracking what happens under real-world pressure — these small steps add up. Every piece of progress on structures like N-Propyl-N-Methylpiperidinium hexafluorophosphate could lead to safer batteries, smarter industrial chemistry, and a smaller environmental bill. Drawing on experience from years watching both lab tests and real product launches, it’s clear the future will continue to hinge on molecules that look simple, but pack a punch once you understand how they work.
Digging into What This Chemical Brings to the Table
N-Propyl-N-Methylpiperidinium Hexafluorophosphate isn’t a name that rolls off the tongue, but it shows up in places you might not expect. This compound landed on my radar during a stint in the battery research lab; I noticed attention always went to those high-profile lithium salts, but this specific ionic liquid played its own quiet yet significant role. Sometimes chemistry just hides its MVPs in the footnotes.
Battery Electrolytes: Lighting the Way
One spot this compound truly shines: battery technology. Many think lithium-ion just needs those well-known salts for smooth sailing, but engineers testing high-performance electrolytes look for something that cuts down resistance and stands up to strong voltages. N-Propyl-N-Methylpiperidinium Hexafluorophosphate brings a unique set of properties—low volatility, high electrochemical stability, and solid ionic conductivity. That translates to safer, long-lasting batteries, especially for electric vehicles where safety concerns stretch past just range anxiety. Research from the Journal of Power Sources (2020) pinpoints this exact ionic liquid as helping to reduce dendrite formation, the dangerous growths that wreck lithium cells and sometimes lead to fires. Lab work shows that with the right blend, battery life ticks upward, and internal short circuits drop sharply.
Electrochemical Devices and Beyond
Supercapacitors and other energy storage gadgets lean on advanced electrolytes for their punch. Some of my early attempts assembling coin cell prototypes showed how much a small tweak in the electrolyte formula swung real performance. This chemical’s broad stability window and strong resistance to breakdown at extreme voltages helps supercapacitors deliver not just energy, but also reliability. Researchers at the Chinese Academy of Sciences found that the ionic conductivity of formulations using N-Propyl-N-Methylpiperidinium Hexafluorophosphate stayed robust above room temperature, leading to higher charge and discharge efficiencies in demo units.
Solvent and Catalysis: Cleaner Chemistry in Practice
Chemists always carry an eye for solvents that can stand up to tough conditions without breaking down or reacting when not wanted. Traditional organic solvents sometimes spark environmental headaches or safety risks. Switching the solvent over to an ionic liquid like N-Propyl-N-Methylpiperidinium Hexafluorophosphate cuts those risks a great deal. Researchers have used it as a “medium” for organic and organometallic reactions, where the unique structure lets reactions proceed at milder conditions and with cleaner results. In my own experiments helping an industrial chemist friend, tests swapping in ionic liquids often cut hazardous waste by half and improved target yields. The Environmental Protection Agency has nudged industries toward greener chemistry, and ionic liquids like this one actually support that goal, guiding companies to cleaner waste streams and energy savings.
Finding the Missing Link: Safety and Market Realities
There’s always a catch. The main concern comes down to cost and scale. These ionic liquids don’t show up cheap or in bulk, so widespread use in large-scale batteries or as universal solvents still faces headwinds. Production costs have tracked downward over the last decade, thanks to demand from energy storage research, but the sticker shock still slows down adoption. Meanwhile, toxicology studies press ahead to make sure that benefits in performance come without hidden health impacts. For instance, German researchers have reported low volatility and fire risk but suggest proper handling protocols just like with other industrial salts.
Looking Forward
Big battery factories keep experimenting with advanced ionic liquids, hoping to balance cost and safety with the push for ever-better performance. Academic labs continue mapping out safer, cheaper synthesis routes. From firsthand observation, the chemistry world seems set on folding these specialized compounds into more real-world devices. The momentum just needs a bit more push from industry and regulators, especially with the global shift toward sustainable energy.
Hands-On Lessons from the Lab
Every chemist remembers the first time they faced a storage dilemma for a specialized salt. N-Propyl-N-Methylpiperidinium Hexafluorophosphate, a mouthful in name and a beast in the bottle, doesn’t forgive shortcuts. Forgetting basic principles of chemical care puts people and research at risk. In my years of working with ionic liquids and specialty salts, careful storage and handling didn’t seem glamorous at first, but every seasoned professional knows overlooked details become accidents that ruin weeks of progress.
Moisture Is the Enemy
These salts pull in moisture from the air like a sponge. Left on a bench, the powder clumps, reacts, and forms impurities that compromise results. At the bench, using desiccators filled with silica gel or freshly activated molecular sieves keeps it dry. Many times I’ve come back to containers not properly sealed, and after only an afternoon, the contents looked different, threatening the whole batch. A dry, low-humidity environment proves essential.
Watch the Temperature
Nobody wants a compound to degrade before it heads to its application. Warm rooms work against long-term stability. Fluctuating temperatures during storage can lead to slow reactions that don’t show up right away, but bite later. I watch for storage rooms where air conditioning isn’t reliable, especially in summer. Stable, cool temperatures extend the lifetime of reagents.
Choose the Right Container
I’ve seen people store hygroscopic salts in glass vials with plastic lids that don’t seal. Eventually, humidity seeps in and corrosion starts. Glass bottles with airtight, chemically resistant caps—PTFE-lined if possible—stand up against reactive materials. Forgetting to label the outside with not just the chemical name, but date received and who opened it, leads to confusion. Good practice keeps chemicals traceable, minimizing mishandling.
Keep It Away from Acids and Bases
Shelving chemicals alphabetically or by hazard class is not just about neatness. Ionic liquids and tetrafluoroborate or hexafluorophosphate salts break down if left near acidic vapors, strong bases, or reactive solvents. I always keep dedicated shelves for salts, away from obvious incompatibles. Mixing signals trouble, sometimes years down the line.
Handling Safety
Nobody in their right mind skips gloves and eye protection. The hexafluorophosphate anion isn’t harmless—fluoride ions do damage. Even minute skin contact has caused colleagues irritation and, in extreme cases, chemical burns. In my own experience, thorough training and working in a well-ventilated fume hood go hand-in-hand. Spills on benches or scales happen suddenly, and knowing your cleanup protocol means you don’t panic. Absorbent pads, neutralizing agents, and emergency eyewash should always be in reach.
Transport and Disposal
Transferring these salts across labs, I use sealed secondary containment. Even a minor spill during transport in a crowded hallway causes unnecessary exposure. For disposal, these compounds belong with hazardous inorganic waste. Ignoring proper waste segregation causes unknown reactions in shared containers. Experience taught me to trust the chemical waste coordinator and check before dumping anything that looks even remotely suspicious.
A Culture of Respect
Complacency tempts many people because nothing bad has happened—yet. Clear labeling, regular checks on container integrity, and open discussions in team meetings keep everyone sharp. I’ve seen groups slack off, only for a small mistake to force an entire shutdown. Respecting the risks means treating every sample as if it could go wrong—because one day, it just might.
Unpacking Its Real-Life Hazards
Handling chemicals like N-Propyl-N-Methylpiperidinium Hexafluorophosphate isn’t just lab work—it’s about respect for invisible risks. The name alone tells you it’s not your average cleaning product. Tucked behind its long title sits a substance both useful in cutting-edge electrochemistry and fraught with potential pitfalls for skin, lungs, and long-term well-being.
Hands-On Work Means Facing Hidden Dangers
Having spent a fair share of time in research settings, I know safety goggles and gloves serve as more than props. This compound, a strong ionic liquid, works well for non-traditional batteries and advanced supercapacitors. But each bottle carries a threat—spills don’t just stain lab benches. Hexafluorophosphate salts raise sharp concerns about accidental skin absorption, eye irritation, and hand-to-mouth exposure. The acute toxicity for humans hasn’t been mapped out to the last decimal point, but related salts cause pain, redness, and sometimes more stubborn effects.
Working with this kind of salt, I always remembered that “inert until spilled” never holds true. Forgotten gloves or mess-ups with ventilation can mean inhaling a dust or vapor that’ll set off sneezing, coughing, and eye-watering. It’s not dramatic; the compound is less harmful than concentrated acids, but its discomfort can linger. Cleaning up without the right gear or disposing it down the drain translates to environmental trouble—hexafluorophosphate doesn’t break down harmlessly. Over time, it could mean tough problems downstream if labs or factories slip up.
Toxicity and Long-Term Impact
Looking at the structure, hexafluorophosphate ions often get flagged for their persistence in water sources. This matters if you believe clean water isn’t negotiable. Testing has shown that related chemicals accumulate and escape filtration, nudging concerns about chronic low exposures that I’d rather not risk. No chemical is harmless just because it’s in small amounts—people have learned that lesson the hard way with things like lead years after initial use.
With basic research, animals and cell lines have shown nerve changes, kidney stress, or blood chemistry swings from comparable salts. It’s not headline-worthy poison, but repeated exposure, even in small doses, adds up—especially where gloves break or filters wear thin. Hexafluorophosphate-based salts react with moisture, sometimes producing tricky byproducts like hydrogen fluoride, which is notorious for difficult burns and slow-to-heal injuries.
How Smart Handling Makes a Difference
It pays huge dividends to keep storage tight, protective gear on, and surfaces clean. Training refreshers matter. I’ve seen vigilance slip in late-night research runs, and one shortcut creates work for weeks—burns, ruined data, and equipment. Chemical fume hoods and eye stations aren’t just box-ticking. They mean nobody’s gambling with nerve health or water quality.
I believe solutions rest on culture and follow-through more than raw technology. Regular checks for leaks, better labels, peer reminders, and waste audits help catch trouble before it snowballs. Manufacturers have started sharing clearer safety sheets and recommended disposal steps. That’s made it easier for those on the ground to spot danger before it’s too late.
Looking Ahead: Industry Responsibility
As batteries and electronics pull in more advanced ionic liquids, companies must build robust routines around purchasing, storing, and disposing of hexafluorophosphate compounds. Better alternatives might surface over time, but until then, eyes on ventilation and careful handling remain non-negotiable. Researchers, tech firms, and universities can’t afford to slack off, not just for legislation, but for peace of mind and public trust.
Understanding What You Buy
I’ve watched research projects stall over one small detail—chemical purity. N-Propyl-N-Methylpiperidinium Hexafluorophosphate, a mouthful by any stretch, drives energy storage experiments and specialty synthesis. It’s not some garden-variety solvent. Laboratories count on consistent results, so tiny differences in purity can throw off tests, sometimes by a mile. When researchers source this compound, they pay close attention to the data sheet. Technical grade may suit pilot-scale work, but for battery development, ultra dry and ultra high purity batches keep side reactions at bay. Phosphorous impurities and water trace amounts make a mess of sensitive electronics or ionic liquids. I’ve seen more than one project pivot just to account for unreliable chemical batches.
Why Purity Levels Aren’t Just a Number
Many chemicals arrive in more than one purity grade. That goes for N-Propyl-N-Methylpiperidinium Hexafluorophosphate as well. I’ve learned the hard way that “good enough” is a risky bet in advanced applications. The difference between 98% and 99.9% purity rises above numbers on a page. Higher water content will degrade lithium-ion cell performance, corrode test rigs, or skew data in catalysis research. Most suppliers spell this out, but buyers shouldn’t skip questions about batch testing and certificates of analysis. Just because a label claims “high purity” doesn’t guarantee the stuff inside matches the ideal. Labs in Japan, Germany, and the United States hold reputation on consistency; they turn to established suppliers for this reason. A casual scan of supply catalogs shows everything from research-grade to electronic-grade options, often at steeply different prices for good reason.
Packing the Right Way
On the shipping side, packaging isn’t just about volume. An acquaintance at a startup once ordered a chemical only to find it arrived in a plastic bottle that compromised stability. Hexafluorophosphate salts stay sensitive to air and moisture; glass vials with tight PTFE seals or aluminum foil lining keep contents dry and intact. Bulk users in manufacturing may want 100-gram or kilo pails, but bench scientists often need 5-gram sample vials. I’ve seen more waste from poorly matched packaging than almost any lab blunder: scientists tossing the whole batch because moisture slipped through. The right container trimmed contamination risk and saved costs as well as frustration.
The Real Costs of Cutting Corners
Every time someone skimps on the prep work—buying the cheapest option, skipping purity checks, ignoring packaging specs—the risks climb. Trace impurities sometimes lead to failed scale-up, safety risks, or regulatory headaches. In a world where research budgets barely stretch, cost-cutting on source chemicals winds up expensive. That said, excessive overspecification burns through funds. The challenge rests in matching purity and packaging to the job, not just chasing the highest number or the biggest drum. Consulting the manufacturer’s data sheet, quizzing the supplier, and researching supplier track records all matter. This diligence feeds into reproducible science and commercial success. I’d rather spend ten extra minutes on procurement than waste months unraveling experimental failures later.
Improving the Supply Chain
Direct conversations with suppliers help both sides get it right. Some manufacturers custom-pack to order, but only if customers speak up early. Listing purity range, water content, and desired milligram-to-kilo packaging in the inquiry can prevent missteps. Chemical supply is rarely perfect, but transparency, informed buyers, and honest suppliers fix most gaps long before they reach the lab bench. In the end, small details in sourcing N-Propyl-N-Methylpiperidinium Hexafluorophosphate shape discoveries, not just the bottom line.