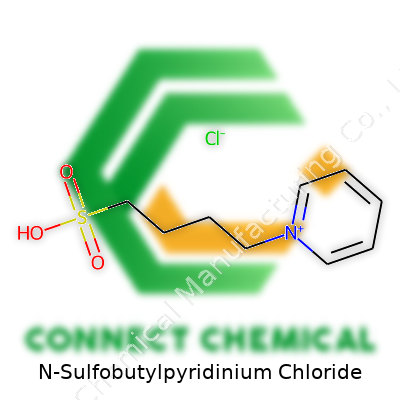
N-Sulfobutylpyridinium Chloride: A Grounded Look at an Important Chemical
Historical Development
Chemicals with pyridinium cores have been used for decades, finding their way from university labs into industry. The development of N-Sulfobutylpyridinium Chloride followed advances in organosulfur and pyridinium chemistry through the middle of the last century. As chemists explored options for introducing sulfonic acid groups into heterocycles, they found that attaching a sulfobutyl chain to pyridinium chloride produced a versatile and stable compound. These efforts aimed to improve solubility in water and stability under various conditions. By the late 1970s, the groundwork was set for this molecule to be produced on a larger scale, offering new potential for technologists and researchers in both academia and business.
Product Overview
N-Sulfobutylpyridinium Chloride gets noticed for its exceptional water solubility, tendency to form stable salts, and compatibility with a range of solvents. Its structure features a pyridinium backbone, which has proven useful for various substitutions, connected to a butyl side chain carrying a sulfonic acid group. These features give the product a unique spot among ionic compounds, where it offers reliable performance as an intermediate in organic synthesis, a coupling agent, and a phase transfer catalyst. Researchers and manufacturers value it most for how easily it mixes into formulations, providing a consistent base for reactions or product development.
Physical & Chemical Properties
N-Sulfobutylpyridinium Chloride generally shows up as a white to off-white crystalline solid. On the lab bench, it dissolves readily in water, creating clear, colorless solutions. It doesn’t give off a strong odor and handles moderate heat fairly well, maintaining structure until temperatures above 200°C. As a quaternary ammonium compound bearing a sulfonic acid group, it acts as a zwitterion in certain solutions but carries a net ionic charge when dissolved. It holds up under acidic and basic conditions, avoiding hydrolysis where many other similar compounds break down. The chloride counterion is easy to displace for more specialized reactions. In direct handling, one finds smooth, free-flowing crystals that don’t clump easily if kept dry.
Technical Specifications & Labeling
Producers usually guarantee purity above 98%, detailing water content, residual solvents, and specific optical rotation. Standard packaging uses airtight, light-protective containers since prolonged UV or moisture exposure can affect quality. Every lot comes with a certificate showing batch number, assay, and heavy metal analysis. European and American labels cover hazards—N-Sulfobutylpyridinium Chloride often appears with GHS hazard pictograms for skin and eye irritation, plus clear directions for ventilation, gloves, and face protection. MSDS data spells out response steps for spills and accidental exposure.
Preparation Method
Chemical production starts with pyridine, which reacts via quaternization with 1,4-butanesultone, inserting a butylsulfonic moiety at the nitrogen atom. The resulting N-(4-sulfobutyl)pyridinium zwitterion gets neutralized with hydrochloric acid, introducing the chloride counterion. This two-step pathway gives strong yields, with most operations running in aqueous or mixed organic solvents to ease separation of byproducts. After neutralization, the solution gets concentrated under vacuum, and crystallization follows as product salts out. Some manufacturers spin-dry or lyophilize, leading to the dry, stable powder ready for end use.
Chemical Reactions & Modifications
The molecule holds up well in standard lab conditions, yet the sultone-derived side chain opens options for further chemical tinkering. Researchers sometimes swap out the chloride for bromide or other halides, broadening utility in anion exchange or electrochemical applications. The pyridinium ring stays fairly inert except under strong nucleophilic substitution or reduction. Sulfobutyl chains can undergo mild oxidation, extending the range of possible modifications. In practical synthetic chemistry, N-Sulfobutylpyridinium Chloride simplifies isolation of specific product phases or boosts yields of polar compounds that stubbornly remain in aqueous layers.
Synonyms & Product Names
N-Sulfobutylpyridinium Chloride shows up in catalogs under names like Pyridinium, N-(4-sulfobutyl)-, chloride; 1-(4-Sulphobutyl)pyridinium Chloride; and sometimes as SBPC. Some suppliers in Asia list it by the English acronym, while European firms stick to the IUPAC naming format. Reference databases and peer-reviewed articles usually settle on the main variant, but those working in the field tend to prefer the shortest possible name for clarity in daily communications.
Safety & Operational Standards
The chemical poses little threat when handled thoughtfully, though direct skin or eye contact will sting and can trigger irritation. I always keep gloves on, work with it under a fume hood, and check that eye wash is close by—especially since the fine powder has a habit of drifting. Emergency protocols stress water rinsing for exposed skin, with medical advice if irritation lingers. As a quaternary ammonium compound, it doesn’t give off toxic fumes but should never be mixed with strong oxidizers or acids. Most institutions where I’ve worked require operators to review updated MSDS sheets and keep an eye on local environmental guidelines, disposing of waste as non-halogenated organic salts.
Application Area
N-Sulfobutylpyridinium Chloride finds its strongest foothold in pharmaceuticals and analytical chemistry. Medicinal chemists look for ways to make active compounds more soluble, and this agent helps. I’ve seen it used to modify drug delivery matrices and create ionic forms of hard-to-dissolve medications. The molecule also works as a phase transfer catalyst—a tool for getting water-soluble reactants to cross over into organic layers where critical reactions finish up. Some labs employ it in capillary electrophoresis, where its charge and hydrophilicity clear the way for sharp analyte separation. In electrochemical devices, it stabilizes electrolyte solutions, making equipment safer and longer-lasting.
Research & Development
Chemists keep exploring new ways to build on the molecule’s framework, often by adding different functional groups to the butyl chain or swapping out the chloride anion. Much research investigates how these tweaks affect solubility, reactivity, and biological compatibility. Large-scale producers push for greener, more cost-efficient synthetic routes—seeking solvents with a smaller environmental footprint and routes that let product recovery get easier and safer. Universities look at the compound’s role in creating new drug salt forms, focusing on reduced toxicity and improved targeting in the body. Electrochemistry groups study its use in smart batteries, hoping for improvements in charge transport and thermal stability.
Toxicity Research
Animal studies indicate low acute oral toxicity, though long-term effects and bioaccumulation remain less fully charted. In my own work, I treat the substance with the caution I’d reserve for any quaternary ammonium chemical. Eye and skin irritancy calls for basic lab protection, and most safety data points to no mutagenic or teratogenic activity at normal exposure levels. Environmental scientists have been examining how it breaks down in wastewater, as persistent ionic compounds raise concern for aquatic organisms. Data so far suggest modest resistance to biodegradation, reinforcing the need for responsible disposal and further investigation of ecological impact.
Future Prospects
New applications keep appearing year by year. As drug molecules grow more complex and water-insoluble, demand for solubilizing agents increases. N-Sulfobutylpyridinium Chloride shows real promise in facilitating targeted drug delivery, especially where traditional excipients fall short. The chemical industry is exploring its use in sustainable manufacturing, where safer, recyclable phase transfer agents are in short supply. Battery research, always on the lookout for ionic liquids and stable salt additives, sees potential in tailored derivatives of this molecule. Tighter safety and environmental regulations mean that producers who develop cleaner processes and better-degrading versions will unlock fresh markets. Researchers continue experimenting with novel modifications and new delivery methods, aiming to open doors in diagnostics, smart devices, and next-generation medicines.
Understanding N-Sulfobutylpyridinium Chloride's Role
Every so often, a chemical pops up that doesn’t get much attention outside a lab, even though it quietly touches the world outside. N-Sulfobutylpyridinium chloride fits that bill. Despite its complicated name, this molecule works in the shadows of industries ranging from pharmaceuticals to electroplating. It’s not just another oddball compound for chemists—it matters for people who rely on cleaner electronics, effective drugs, and improved industrial processes.
The Science Behind the Scenes
This compound is a quaternary ammonium salt — think of it as a type of chemical helper that tends to pop up where reactions need a boost or special characteristics. In electroplating, labs and factories need metals to coat surfaces smoothly and evenly. Without a key additive like N-Sulfobutylpyridinium chloride, coatings often turn out patchy or rough. That spells trouble in fields where a single glitch sends a smartphone, vehicle circuit board, or medical device to the recycling pile.
For at least a decade, research has tracked how N-Sulfobutylpyridinium chloride makes copper plating smoother and more stable. In some electronics manufacturing, even a tiny amount of impurity creates defects that cost thousands or millions to fix. People rarely see these details, but the price for skipping quality shows up in shorter device lifespans and higher repair rates.
Reaching into Medicine
Lately, the pharmaceutical industry has chased ways to improve how drugs dissolve or absorb in the body. Salts like N-Sulfobutylpyridinium chloride act as solubilizers—helping stubborn ingredients mix with watery solutions. These ingredients can give drug makers a way to get reliable, predictable medicine into systems where every dose counts. Some generic medications can't mirror expensive brand-name drugs without these helpers. That gives this compound a behind-the-scenes role in broadening patient access to crucial therapy.
Safety and Environment
Any compound used in industry stirs up questions around safety and disposal. N-Sulfobutylpyridinium chloride highlights how regulatory oversight matters. I’ve seen facilities where chemists pay careful attention to how much they use and how they clear leftovers. The move toward green chemistry shows up every year in tighter standards and environmental reports. In Europe and much of Asia, manufacturers follow strict protocols to keep chemical runoff away from public water systems. Without these safeguards, communities could face contaminated drinking water or harmed wildlife.
Where’s the Road Ahead?
Lower-impact chemicals offer promise, and newer research already explores ways to match the performance of N-Sulfobutylpyridinium chloride with fewer risks. Scientists constantly run laboratory experiments to test substitutes that break down more quickly or lack long-term toxicity. In the meantime, education goes a long way—technicians need practical training and real incentives to cut hazardous exposure. If I learned anything in industry labs, the smallest details on a chemical safety sheet matter just as much as a fancy new process breakthrough.
The chemical itself might not make headlines, but its uses tie directly to product safety, affordability, and how well we protect our environment. For folks who care about what ends up in their gadgets or medicine cabinets, this compound is worth a closer look.
What Makes N-Sulfobutylpyridinium Chloride Useful?
N-Sulfobutylpyridinium chloride doesn’t often make headlines, but chemists and engineers rely on it for its toughness in specialized settings. Its unique molecular structure lets it serve as a highly efficient ion-pairing agent, and that translates to real value in many labs. People started to take more notice once pharmaceutical analysts looked for better ways to run high-performance liquid chromatography (HPLC) separations. During my own work with HPLC, adding this salt elevated sensitivity for charged analytes, especially basic compounds that stubbornly resisted resolution.
Chromatography: Raising the Bar for Drug Analysis
In pharmaceutical quality control, there’s a constant battle to identify and measure tiny amounts of impurities—or the main active compound itself. N-Sulfobutylpyridinium chloride helps separate molecules that would otherwise overlap. This comes from its role as an ion-pairing reagent in reversed-phase liquid chromatography. For molecules that aren’t easy to tame, especially cationic drugs, this compound lets analysts push the boundaries of resolution. In regular practice, this means regulators get more precise data about the medications they approve. It’s not glamorous, but this kind of accuracy makes a real difference in patient safety, which explains why regulatory agencies keep an eye on these tools.
Electrochemical and Analytical Testing
The same property—effective ion pairing—pays off outside of pharma labs. Researchers in electrochemistry use N-Sulfobutylpyridinium chloride as a conducting electrolyte in sensors and separation techniques. It keeps charged molecules steady and boosts signal strength for measurements. That comes in handy when tracking ions in water or monitoring pollutants in environmental testing. During my time working with water analysis, swapping in this compound delivered cleaner, stronger signals with less background interference. The data just looked sharper, and the numbers spoke for themselves.
Dye and Pigment Processing
Textile and ink manufacturers love dyes that stay bright and stick to fiber without bleeding. N-Sulfobutylpyridinium chloride enters this space as a dispersant and dye-migration control agent. Its sulfonic acid group helps bind dyes to fabrics and improves stability during washing or exposure to light. This translates into longer-lasting color on everyday products. Some factories that switched over saw a measurable drop in dye loss—data that points to cost savings and less waste water pollution.
Bridging Gaps in Drug Discovery
Drug chemists hunting for next-generation molecules need tools for extracting, separating, and analyzing new compounds. N-Sulfobutylpyridinium chloride often lands in sample preparation kits for these jobs. Used in solid-phase extraction, for example, it helps isolate ionic drugs and metabolites from complex fluids. This speeds up the testing cycle and means fewer false negatives in research datasets. Faster, more reliable analysis streamlines R&D and nudges promising drug candidates toward market.
Paths Forward
The safety data behind N-Sulfobutylpyridinium chloride remains important. My own approach includes keeping up with regulatory reviews, especially around pharmaceutical use. Companies could push for greener synthesis methods or more biodegradable alternatives to lower environmental risk. Open discussion inside scientific circles helps—if teams share process improvements or alternate reagents, whole industries can adopt safer, more efficient workflows. As demand for sharper testing and stronger dyes grows, expecting innovation in chemicals like this seems like a smart bet.
Understanding the Chemical’s Profile
N-Sulfobutylpyridinium chloride turns up in a surprising number of modern applications. Labs use this compound in analytical chemistry, and some sectors look at it as a surfactant or electrochemical agent. Many people have never heard of it, but the real point here focuses on everyday safety for anyone who handles compounds like this.
Potential Risks in the Workplace
Sometimes, chemicals sound intimidating simply because of their long names. Risk depends on how a substance behaves in your hands, your skin, and your environment. N-Sulfobutylpyridinium chloride, like many pyridinium salts, won’t leap out of the bottle to harm you. Still, its safety profile depends on concentration, exposure time, and basic chemical hygiene in the workspace.
People recognize that manufacturers label this compound with hazard statements warning about skin and eye irritation. Direct contact could sting; breathing in dust or powder might bother your airways. That reminds me of other lab work, where you learned fast not to touch your face until you washed your hands. Personal protective equipment, or PPE, isn’t just a rule—it keeps small accidents small.
Why Safe Handling Matters
Safety isn’t just about ticking boxes on a safety data sheet. It’s about trust in your team, feeling confident you’ll get home healthy. A careless moment can force a shutdown or cause a trip to urgent care. In almost every lab I’ve worked, gloves and goggles aren’t up for debate. Even if something looks like plain salt, nobody guesses with chemicals.
The European Chemicals Agency classifies N-Sulfobutylpyridinium chloride as irritating to skin and eyes. If spilled, it can get messy and potentially corrosive—especially in higher concentrations. Swallowing or inhaling dust can escalate from mild irritation to serious discomfort, or worse. This stuff isn’t cyanide, but treating it casually just invites problems.
Building a Culture of Caution
Many workplaces take shortcuts, usually out of habit or because they’ve never seen a problem up close. The truth is accidents rarely warn you. Spills, splashes, or carelessness build up as stories over the years, shaping better habits. I always preferred the teams that double-checked labels before pouring, used fume hoods for powder weighing, and stored chemicals tightly sealed.
Strong habits keep a minor compound from turning into a serious headache. The health and safety executive in the UK and OSHA in the US both suggest the same: keep good storage, make sure the ventilation works, and use the right gloves for organics. Information from MIT’s Environmental Health and Safety office lists nitrile gloves, chemical splash goggles, and full lab coats for substances with similar risk.
Solutions for Better Safety
Training never stops. It’s not enough to read a safety sheet on the first day and push it to the back of a drawer. Periodic refreshers about what to do with spills, accidental exposure, and chemical waste pay off. Emergency showers and eyewash stations make sense no matter how “benign” a chemical seems.
In practice, reducing dust creation and avoiding contact with skin and eyes means thinking about workflow. Opening containers over a tray, cleaning counters at the end of the day, and storing even “safe” chemicals securely makes the difference. When uncertainty pops up, ask the expert or review updated chemical data. Handling N-Sulfobutylpyridinium chloride safely isn’t rocket science—it’s just basic self-care, experience, and respect for the chemistry.
The Structure Up Close
N-Sulfobutylpyridinium chloride stands out for more than its tongue-twisting name. Break it down, and you get a molecule based on pyridine—a simple aromatic ring with a nitrogen atom swapped in for one carbon. This ring structure gives it a major dose of chemical versatility, familiar to chemists working with basic catalysts or pharmaceutical intermediates.
But N-sulfobutylpyridinium chloride isn’t just pyridine. The core gets a big modification: a “butyl” chain—four carbons long—gets anchored to that nitrogen. The twist is in the chain’s ending. The butyl's terminal carbon carries a sulfonate group, a cluster packing a sulfur atom double-bonded to two oxygens and single-bonded to another oxygen carrying a negative charge. Line that up with the nitrogen in the pyridine ring; the full name tells the story: the butyl is “sulfobutyl”, and it’s tied to the nitrogen spot. That gives the molecule a positive charge on the nitrogen and a matching chloride counterion that keeps the whole thing stable. Picture it as a pyridine ring with an extra appendage, balancing electrical charges.
Why the Structure Holds Value
Looking at the molecule’s architecture, you start to see why it catches the eye of scientists and manufacturers alike. Sulfonate groups love water, so this derivative dissolves more willingly than plain pyridinium salts that lack such chains. In the lab, this can be a game changer. I recall trying to dissolve stubborn aromatic salts in my early days—it took a good deal of coaxing and sometimes didn’t work at all. Add sulfonate tails, and things go smoothly in water or saline. That boost in solubility means you can use it in a range of environments—even inside the human body for medical research.
It’s not just about getting it to dissolve. The positive charge on the nitrogen and the negative charge on the sulfonate group often encourage attractive interactions with other molecules. This makes N-sulfobutylpyridinium chloride useful in extraction chemistry, where you want to separate out specific compounds, and in formulation science, where you control how ingredients mix and react. In pharmaceutical development, these properties support delivery of active compounds by helping them travel across cell membranes or stay stable under stress.
Supporting Evidence and Real-World Applications
Scientific literature points to the growing use of charged aromatic rings in green chemistry. One research paper in Green Chemistry (Royal Society of Chemistry) describes how sulfonated pyridinium salts serve as ionic liquids—a class of compounds prized for low volatility that cuts down on harmful emissions in chemical processes. At industrial scale, these salts often step in as phase transfer catalysts, moving reactants between oil and water phases to speed up reactions and reduce waste.
Regulatory bodies like the European Chemicals Agency track new molecules for environmental safety and health impact. In their reports, N-sulfobutylpyridinium chloride features as a lower-risk choice compared to less soluble or more toxic salts, because it tends to stay dissolved and gets flushed out more reliably by biological and water treatment systems. In direct applications, you’ll find it in buffer solutions and as a component in lab kits for separating DNA and proteins.
Challenges and Room for Growth
Chemical stability matters. While the sulfonate group adds benefits, it can react with strong acids or bases, and the chloride ion sometimes competes in side reactions—not ideal for finely controlled syntheses. Improved synthesis pathways that yield higher purity and fewer byproducts keep attracting attention from chemical engineers and purification specialists. The future might see even more tailored versions—perhaps with different sulfonate lengths or alternative counterions—targeting problems like drug solubility and advanced battery electrolytes.
Why Storage Really Matters
N-Sulfobutylpyridinium chloride doesn’t hop around the headlines, but anyone who handles chemicals knows: a sloppy shelf can turn a safe product into a headache or a hazard. Labs and factories, large or small, face risks if storage gets sloppy. Most folks in chemical jobs have run into a bottle past its prime, maybe with a crust forming or some funky smell that means trouble. Clean practices at the core level not only prevent waste, but also protect workers from dangerous reactions.
Temperature and Placement: Getting the Basics Right
Shelf life isn’t just about the clock. N-Sulfobutylpyridinium chloride likes it cool and dry. For decades, chemists and lab techs have followed this simple rule: chemicals that dislike moisture and heat go into cabinets away from sunlight and heat vents. Avoiding sunlight keeps the product stable. Air conditioning and controlled ventilation cut down the risk of a temperature spike. Refrigeration’s rarely needed unless the label says so, but check for condensation inside and wipe down bottles before putting them away.
Moisture and Contamination: Quiet Dangers
I’ve seen more than one new hire crack open a cap with damp gloves, then wonder why powder sticks to the inside later. Even a little water in the mix can mean degradation or even a dangerous reaction. Keep the container tightly sealed after every use. Don’t set the bottle near sinks or in humid rooms, even for an hour. Desiccators or sealed boxes with drying agents give an added layer of dryness for long-term storage. Once, I found a stash of bottles under a leaky window; weeks later, labels had turned gummy and the material lost half its strength. Dampness ruins shelf life and introduces impurities you can’t always spot.
Safe Labeling and Segregation
Anyone who’s sorted through shelves in a busy lab knows how easy it is to grab the wrong container. Use clear, large labels, and update them if the contents get repackaged or diluted. Never store N-Sulfobutylpyridinium chloride alongside reactive chemicals, like strong acids or bases. Cross-contamination risks shoot up if storage gets crowded or unorganized. I’ve seen a lab shut down for days after a mislabeled jug ended up next to something incompatible, causing a spill that prompted an evacuation.
Incident Response Starts With Storage Smarts
Personal experience and more than a few stories around the coffee machine confirm this: good storage stops small mistakes from becoming disasters. Keeping proper spill kits close by, knowing who’s responsible for routine checks, and writing down inventory details all add up over time. If a container looks off—cracked lid, weird smell, discoloration—dispose of it properly instead of taking a chance. Trained staff spot the signs early and handle problems before they spread.
Supporting Safety With Training and Mindset
Regular refresher training makes storage rules stick, especially for teams facing high turnover. New folks sometimes rush and skip best practices. Leaders set the tone by doing spot checks and showing everyone what right looks like. Safety culture grows from constant reminders, not once-a-year lectures. Labs that manage storage tightly report fewer accidents and waste fewer chemicals, protecting staff and the budget.
Looking Ahead: Simple Steps For Better Practice
No room for shortcuts in chemical storage. Give N-Sulfobutylpyridinium chloride a spot on a clean, climate-controlled shelf, and you’ll avoid most trouble. Take labeling seriously, review storage spots often, and keep humidity at bay. Mistakes cost more than time—they put people and projects at risk. Experience proves that smart habits make all the difference in the long run.