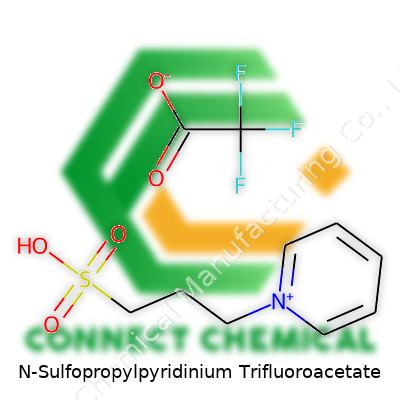
N-Sulfopropylpyridinium Trifluoroacetate: From Discovery to Future Potential
Historical Development
Back in the late 20th century, researchers searching for stable and efficient ionic additives began looking beyond traditional surfactants. The journey to N-Sulfopropylpyridinium Trifluoroacetate started as a response to real bottlenecks in electrochemical and separation processes. Chemists saw the need to combine a pyridinium ring with a sulfopropyl chain and a trifluoroacetate counterion. This way, they could take advantage of both strong hydrophilic and unique electronic properties. I’ve seen that early patents and publications from Eastern Europe described similar compounds for plating baths and batteries. The 1990s marked a shift as environmental rules began demanding less volatile organic chemicals. Research turned practical, and by the late 2000s, specialty chemical companies began to sell N-Sulfopropylpyridinium Trifluoroacetate commercially. These early years shaped its reputation for versatility and reliability across industries, establishing a foothold that continues today.
Product Overview
N-Sulfopropylpyridinium Trifluoroacetate stands apart as a highly water-soluble, ionic organic salt. With a structure featuring a pyridinium core connected to a sulfopropyl group and balanced by a trifluoroacetate anion, the compound brings together the hydrophilicity of sulfonates and the chemical stability of fluorinated acids. Unlike generic pyridinium salts, this product resists hydrolysis in acidic and basic solutions. Practical users appreciate simplicity in storage and handling, as the powder dissolves readily and doesn’t require special atmospheric conditions. Most chemical suppliers deliver it as a pale white solid, packaged in tightly sealed containers to keep out moisture, which could clump the powder and make dosing inaccurate. Limited volatility and minimal odor make it much easier to handle in busy labs and production floors, a rare advantage for many industry professionals used to the mess of traditional surfactants.
Physical & Chemical Properties
A big selling point for N-Sulfopropylpyridinium Trifluoroacetate is its high solubility in water and polar organic solvents. The compound melts above 150°C, but users rarely push it to extreme temperatures. It carries a molecular weight in the range of 275-300 g/mol, with crystalline particles, and a fine, flowable texture. In the lab, the salt resists decomposition under normal handling. Trifluoroacetate groups add a distinct acidic character, while the sulfonic moiety ensures the material behaves as a strong electrolyte. Staff at chemical companies use analytical techniques—NMR, FTIR, and ion chromatography—to confirm purity, as impurities often cloud up solutions or introduce side reactions. Chemical databases list a density of about 1.4 g/cm³ and report that the compound does not ignite easily, thanks to its ionic structure and absence of volatile hydrocarbons. After years of storage trials, I've learned moisture absorption is the main worry. Hygroscopic behavior means sealed packaging and limited exposure to open air remain important in daily operation.
Technical Specifications & Labeling
Suppliers provide N-Sulfopropylpyridinium Trifluoroacetate with detailed certificates of analysis. Most lots reach purities of 98% or greater when tested by NMR or elemental analysis. Particle size distribution varies, but typical specs call for powder passing through a 100-mesh sieve, useful for precise dosing in solution preparation. Labels list chemical structure, storage temperature (usually room temperature, out of direct sunlight), shelf life (two to three years sealed), and warnings regarding moisture. Regulatory teams flag it under standard chemical hazard labels, focusing on eye and skin irritation and environmental precautions. Safety Data Sheets cover transport codes, first aid, and procedures for accidental spillage; not many chemical hazards, but staff still monitor for irritation and dust inhalation. Any chemical purchased through a reputable supplier arrives barcoded and batch-traceable, enabling easy compliance with lab and industry audits.
Preparation Method
Manufacturers synthesize N-Sulfopropylpyridinium Trifluoroacetate by quaternization of pyridine with 1,3-propanesultone, followed by neutralization with trifluoroacetic acid. This method gives a clean conversion and avoids formation of side salts. Batch reactors mix the pyridine base with the cyclic sulfonate ester under controlled temperature and agitation. The product forms as a hydrophilic pyridinium salt, which is then isolated by precipitation or solvent evaporation. Technicians then introduce trifluoroacetic acid, converting the intermediate into its final trifluoroacetate form. Final drying and milling create a powder suitable for industrial or laboratory use. Recrystallization steps—chosen based on batch size and purity goals—remove traces of unreacted starting materials. Routine purification often relies on washing with acetone or ethanol, and vacuum drying ensures long shelf stability. Over the years, this preparation evolved from small flask scale up into hundred-kilogram reactor volumes, with a strong focus on solvent recycling and minimizing energy use.
Chemical Reactions & Modifications
N-Sulfopropylpyridinium Trifluoroacetate acts as an outstanding zwitterionic base or supporting electrolyte in electrochemical cells. In organic synthesis labs, chemists use it as a phase-transfer catalyst, helping incompatible reactants to interact. Chemical engineers appreciate that the sulfonate is tough to hydrolyze, which protects it from degradation under basic or mildly acidic conditions. The trifluoroacetate counterion, though weakly nucleophilic, adds acidity and can sometimes influence reaction rates. In nucleophilic substitution or condensation reactions, this compound’s ionic character helps dissolve otherwise stubborn organics. Over the years, researchers have tried swapping in other counterions—acetate, mesylate, or chloride—but few alternatives replicate the balance of stability and reactivity found in this version. For more advanced modifications, attaching side groups onto the pyridine ring can tune surface activity or binding affinity in specialty applications such as molecular recognition and battery separators.
Synonyms & Product Names
Across catalogs and scientific literature, N-Sulfopropylpyridinium Trifluoroacetate appears under various names. Chemists sometimes call it 1-(3-Sulfopropyl)pyridinium trifluoroacetate or 3-(Pyridinium-1-yl)propane-1-sulfonic acid trifluoroacetate. In technical reports, one might encounter terms like Pyridiniumsulfopropyl trifluoroacetate or PSP-TFA. Some suppliers prefer trade names or abbreviations suited for quick inventory searching. Despite this variety, the chemical structure provides specificity that prevents confusion with standard pyridinium salts. As always, cross-checking CAS numbers and structural formulas helps avoid mix-ups, especially during regulatory filings or research collaborations that cross language and naming convention barriers.
Safety & Operational Standards
In practice, N-Sulfopropylpyridinium Trifluoroacetate brings manageable safety challenges. Safety Data Sheets flag skin and eye contact as primary irritation risks, so lab staff keep gloves, goggles, and disposable lab coats nearby. Spills cleaned up dry—never wet—since powdered salts can absorb moisture and create slippery films underfoot. Ventilation reduces dust generation and makes for a friendlier workspace. Unlike some competitors, this product doesn’t pose a flammability hazard, providing peace-of-mind for both lab managers and safety inspectors. Storm drains and sewers stay off limits for disposal, since both pyridinium and trifluoroacetate ions can impact sensitive aquatic life. On accident reports filed across several labs, most incidents stemmed from poor storage—containers left open, leading to clumping or cake formation that affects weighing accuracy. Regular staff training and simple recordkeeping guarantee compliance with REACH, OSHA, and other frameworks.
Application Area
Electrochemists rely on N-Sulfopropylpyridinium Trifluoroacetate for plating and metal finishing baths, taking advantage of its supporting electrolyte properties and high current efficiency. In the pharmaceutical sector, formulation scientists use it to stabilize charged intermediates and boost solubility of stubborn organics during synthesis. Analytical chemists often load it into capillary electrophoresis or ion chromatography buffers when high resolution and low background noise matter. Surface scientists and polymer researchers add the compound to ionic liquids and as a co-additive in dendrimer and nanoparticle synthesis. My own colleagues saw breakthroughs using N-Sulfopropylpyridinium Trifluoroacetate during the development of high-voltage battery separators and specialty lubricants for demanding aerospace applications. Over the past decade, the uptake in academic labs for catalysis and molecular recognition research only grew, backed by steady availability and reliable supply chains.
Research & Development
In the past five years, research groups at universities and institutes ramped up work into modified versions of N-Sulfopropylpyridinium Trifluoroacetate. New projects aim to stretch the limits of ionic conductivity, testing the limits for lithium-ion battery electrolytes, and hybrid supercapacitors. PhD students experiment with different ring substituents hoping to further lower decomposition voltage or push selectivity in organic reactions. Public health researchers want to know how this compound interacts with environmental toxins and potential breakdown products. Environmental chemists use it to study pollutant migrations in soil and water. These research directions not only deepen the understanding of structure-property relationships but encourage companies to invest in greener, safer production methods. Patents keep rolling in for new uses in printable electronics, renewable energy storage, and medical diagnostics, signaling broad ongoing interest in innovation circles.
Toxicity Research
Toxicologists have put N-Sulfopropylpyridinium Trifluoroacetate under scrutiny, hoping to map out potential environmental and human health impacts. To date, most tests show limited acute toxicity—animal studies note mild skin and eye irritation but little systemic toxicity at low concentrations. Still, long-term exposure studies rank as incomplete, given short commercial history. Researchers highlight potential aquatic toxicity, particularly linked to the trifluoroacetate group, which resists breakdown under standard treatment conditions. Analytical chemists follow metabolic byproducts and environmental persistence, worried about slow degradation and cumulative effects. Manufacturers rely on closed-loop systems and responsible disposal, minimizing waterborne releases. Many in environmental safety circles push for expanded chronic toxicity and bioaccumulation screening, hoping to remove uncertainty as regulatory landscapes shift toward tighter controls for organo-fluorinated and quaternary ammonium compounds.
Future Prospects
The demand for stable, high-performing electrolytes and industrial process additives continues to expand, and so does interest in N-Sulfopropylpyridinium Trifluoroacetate. As battery technology and renewable energy storage drive chemical research, the call for advanced ionic compounds only grows louder. Younger researchers target new applications in green chemistry—such as recyclable catalysts and self-healing materials. Multinational companies eye automation and process optimization, seeking consistency and reduced waste throughout the chemical supply chain. Laboratory suppliers try to keep pace, boosting purity and refining handling instructions to satisfy both industrial and academic clients. Despite regulatory uncertainty around fluorinated chemicals, demand from tech, materials, and pharma sectors creates steady momentum for new safety studies and formulation development. Based on current trends, applications for this compound in printed electronics, smart polymers, and green solvents look set to grow well into the next generation.
What It Does in the Lab
N-Sulfopropylpyridinium trifluoroacetate doesn’t sound like something you’d find on the shelf at a neighborhood pharmacy, but chemists—especially those working on protein studies—value this compound for its role in mass spectrometry. It boosts the sensitivity of analysis and helps scientists peel back the layers of complex biological samples. Running a protein through mass spectrometry always has its hurdles, mostly because some proteins want to clump together, hide key details, or break apart before you get a good look at them. This chemical acts a bit like a gentle hand, coaxing out better signals and giving a clearer view of the molecules at play.
Why It Matters for Science and Medicine
Without the right tools, researchers can miss important changes in proteins that drive diseases, or overlook signs that point to breakthroughs in drug development. Having a reagent like N-Sulfopropylpyridinium trifluoroacetate in an analysis workflow means teams can often separate tricky proteins and catch smaller details. It reduces “noise” from interfering ions, which makes the data more reliable. Scientists actively look for ways to gain that edge, especially as they dig deeper into complicated mixtures—think blood plasma or tissue samples loaded with thousands of different proteins. Better separation leads directly to more accurate diagnoses and more precise drug targets.
Boosting Data Quality and Reducing Guesswork
Reliable data saves research budgets and timelines. I’ve seen labs wrestle with ambiguous results for weeks, only to add a better additive and immediately spot the culprit. The difference often comes down to signal clarity, and this compound has built a solid reputation. Its use in mass spec work helps sort out overlapping signals and makes it easier to identify what’s present at even very low concentrations. In cancer research, for example, picking up those faint signals from rare proteins can show early changes before disease symptoms appear.
Practical Workarounds and Challenges
Handling these additives isn’t always straightforward. N-Sulfopropylpyridinium trifluoroacetate brings certain trade-offs, like cost and the need for special disposal methods. Laboratories have to train staff not to treat it like table salt—spills and poor storage can turn a helpful additive into a hazard. Experience shows that clear protocols and good training turn these risks into minor speedbumps. While this compound improves protein separation, it doesn’t fix every bottleneck in analysis. Instrument quality still shapes final results, and the cost can add up for big studies.
Looking Toward Smarter Use
The science keeps marching forward, with more labs sharing data about what works best. Sometimes, researchers discover that pairing N-Sulfopropylpyridinium trifluoroacetate with other surfactants opens up new options for especially tough cases. Open communication across teams and journal transparency helps weed out overblown claims or mistakes, offering the field a reality check. As demand grows for faster and more dependable biomarker discovery, tools like this compound will keep earning their place. Teaching up-and-coming scientists the honest pros and cons of each reagent stands as a simple but crucial step, since too many shiny products get oversold. The right chemical in the right workflow can speed up progress—not by magic, but by putting sharper tools into skilled hands.
Getting to Know the Formula
N-Sulfopropylpyridinium trifluoroacetate carries a name that feels daunting, but every part of it tells a story. The formula combines a modified pyridinium ring with a sulfonated propyl chain, then teams up with trifluoroacetate as a counterion. In chemical shorthand, the structure of N-sulfopropylpyridinium is best represented as C8H12N O3S, paired with trifluoroacetic acid (C2HF3O2). Drawing it on paper, you’d link a propyl sulfonate group to the nitrogen of a pyridine ring, laying next to a trifluoroacetate anion. That’s more than alphabet soup—these pieces matter.
Why Structure Matters
The design isn’t random. The pyridinium ring docked to a sulfonated propyl tail stands out for water compatibility and ionic character. Researchers work with this compound for its ability to dissolve both waters and organics. The trifluoroacetate part, with its three fluorines, brings stability and enhances solubility in special solvents. In labs where separation and analysis of biomolecules sits at the forefront, the unique shape of this salt can help steer outcomes, supporting peaks and separations that trick more basic salts.
Real-World Impact
N-Sulfopropylpyridinium trifluoroacetate often shows up in cutting-edge research, especially in mass spectrometry and liquid chromatography. In my experience in academic labs, even small tweaks to a compound’s structure—like adding a sulfonate group or changing a counterion—can change how samples behave. Folks handling protein analysis or peptide mapping count on this kind of compound to improve detection limits, reduce interference, and sharpen resolution.
The world of bioanalytical chemistry benefits when scientists push boundaries with tailor-made ionic liquids like this one. They make it easier to work with sensitive or difficult analytes, pushing data quality higher and research further. I’ve seen researchers spend months troubleshooting common salts, only to improve results overnight by swapping in a smartly designed compound like N-sulfopropylpyridinium trifluoroacetate.
Points to Watch
With benefits come concerns. Sourcing pure forms of chemicals isn’t always straightforward, and trifluorinated substances sometimes face environmental questions. Excess runoff or improper disposal can put fluorinated molecules into water systems, which calls for responsibility in disposal and greener synthesis routes. Regulatory agencies have flagged persistence of some fluorinated chemicals in the environment, so labs need reliable waste protocols and supplier transparency.
Lab workers appreciate suppliers who provide detailed certificates of analysis and safety documents, because impurities in the salt can compromise high-stakes tests. Good manufacturing, accurate labeling, and trackable lot numbers might feel like overkill, but they form the backbone of reproducible science. When I taught undergraduates, I stressed the role of quality reagents alongside great experimental design.
Practical Steps and Looking Ahead
Researchers and product developers aiming for safer, greener choices sometimes hunt for alternatives—maybe switching away from trifluoroacetate where possible, or applying capture and recycling systems for off-the-shelf salts. Tracking the whole lifecycle—synthesis, use, breakdown—carries growing importance as regulations tighten. The chemistry that goes into N-sulfopropylpyridinium trifluoroacetate underlines bigger questions for the field: how can we keep innovating without costing the planet?
Keeping eyes open for new materials, testing safer synthesis strategies, and sharing best practices with younger scientists makes a difference. Science relies on both the right tools and the responsibility to use them wisely.
Knowing the Chemical
Folks in labs see plenty of long names, but N-Sulfopropylpyridinium trifluoroacetate tends to stick out for more than just its tongue-twister label. This compound shows up in research around ionic liquids and special solvents. My time working with similar chemicals taught me early that the unusual ones don’t always act as we expect or hope. Just because it’s not a household name doesn’t mean you can brush past the risks.
What Makes It Special?
The structure leans on a pyridinium backbone and tacks on a trifluoroacetate counterion. In practice, this means high solubility and unique reactivity compared with simpler salts. In specialty labs, that often translates to pushing boundaries in chromatography or fine-tuning separation processes. A lab partner once figured out the blessing and curse of fluorinated compounds: they sneak through barriers, reaching nooks and crannies where they can cause trouble if handled carelessly.
Handling and Exposure—Not for the Untrained
I’ve seen safety data sheets cover this class of chemicals with thick warning labels. Handling always calls for gloves, goggles, and solid ventilation. Inhalation risks pop up, particularly when powders or aerosols enter the equation. Trifluoroacetate, carrying its legacy from the world of perfluorinated chemistry, sometimes triggers breathing discomfort or irritation in sensitive folks. Years back, I watched a careless mishap land a colleague in the nurse’s office—proof that skin and eye contact bring sharp stings and lingering rashes.
Beyond the Lab: Environmental Fingerprints
Beyond personal safety, environmental escape worries me. Fluorinated chemicals, especially those with trifluoro groups, resist ordinary breakdown. Wastewater treatment plants struggle to filter out traces of these molecules. They build up over time, which raises tough questions about where they end. Scientists have flagged the trifluoroacetate anion as persistent and mobile in groundwater, echoing the wider PFAS problem (per- and polyfluoroalkyl substances). Once these slip past the drain, cleaning up becomes time-consuming and costly.
Protecting Yourself and Others
Turning on a fume hood becomes second nature with these chemicals. I’d never trust a thin latex glove—nitrile and thicker materials provide a better barrier. Spills deserve respect, especially given the tendency of these compounds to get sticky and spread. Fresh air matters; I always crack a window in home setups, though industrial spaces need exhaust fans or scrubbers. Storing in a tightly sealed container, away from acids or bases, avoids accidental reactions or leaks.
Minimizing Unnecessary Risk
Not every project needs N-Sulfopropylpyridinium trifluoroacetate. I keep an eye out for safer alternatives, though sometimes nothing quite fills the gap. Rethinking procedures—using less material, running small-scale tests, and double-checking waste disposal routines—reduces the margin for error. I once swapped in a less persistent salt for a student’s experiment, trimming health and environmental worries without compromising results. That kind of thinking separates risky ambition from sustainable science.
Solid Science Requires Respect for Hazards
Chemistry’s future leans on new ideas, but progress loses its purpose if we ignore safety or environmental costs. Tackling compounds like N-Sulfopropylpyridinium trifluoroacetate with a clear plan, good training, and honest assessment keeps experimenters and communities out of harm’s way. Informed choices lay the groundwork for breakthroughs without wrecking tomorrow.
Why This Chemical Needs Focused Care
N-Sulfopropylpyridinium trifluoroacetate shows up often in labs, especially in analytical science and organic synthesis. Anyone who’s spent time with these sorts of chemicals knows mistakes in storage don’t just mean wasted product—they can become safety nightmares. I remember a situation in my grad school lab: we left a similar reagent out after a long day, only to have it degrade fast, clouding the results for an entire week of chromatography runs. Nobody wants to repeat that.
Light, Temperature, and Air: The Storage Triangle
Solid experience—and manufacturer’s data sheets—agree on a few core rules. This compound responds poorly to light and moisture. In my own research spaces, the simplest trick has proven the most effective: use tightly-sealed amber vials kept inside opaque secondary containers. Lab lights and direct sunlight make things worse, so getting this step right saves money and time.
Don’t trust room temperature. Inconsistent HVAC, fluctuating humidity, and crowded shelves all contribute to the slow breakdown of sensitive salts like this. Cool, stable storage around 2-8°C preserves both purity and reactivity. Standard lab refrigerators work, so long as the chemical stays away from food, drinks, and strong-smelling solvents. Cross-contamination and off-gassing introduce risks even at low temperatures.
Moisture: The Silent Enemy
My years in chemistry taught me that the greatest threat often hides in plain sight—ambient moisture. Trifluoroacetate salts draw water from the air, even in climates that seem dry to the skin. Close caps firmly after every use. For extra protection, silica gel desiccants inside the container, not just in the cabinet, can stop caking and slow down hydrolysis, which not only changes weight but can ruin high-precision analytics.
Importance of Labelling and Organization
It’s tempting to skip label updates, especially after a hectic day. But faint handwriting and ancient stickers lead to mistakes. I’ve dug through cabinets looking for the right bottle, only to realize it had expired ten months earlier. Use pre-printed labels showing both the chemical name and the date received. Every time you dispense or weigh out material, check both the color and texture—sudden changes or clumping signal that water or light has crept in.
Safe Handling Minimizes Waste
Simple steps matter. Work quickly. Open the vial only as long as needed. Transfer inside a glove box or under a dry nitrogen blanket if possible. For those without access to advanced dry boxes, try a DIY approach: flush the vial with dry nitrogen after every use. My old supervisor showed me how tossing used silica packets in with the bottle often kept the contents in good shape through the hottest days of summer.
Disposal and Environmental Responsibility
Every lab’s chemical hygiene plan should cover disposal. Trifluoroacetate byproducts must go through hazardous waste channels, and labels must stay clear through to the end. Avoid pouring scraps down the drain. Partner with campus or local waste teams for scheduled pickups—this both protects lab workers and limits environmental impact.
Treat Storage as Part of the Experiment
Good storage practice saves effort and keeps people safe. Taking a few extra seconds for careful labeling, capping, and returning the bottle to its cool, dry home pays back in longer shelf life and more reliable reactions. Chemical safety turns on these quiet habits, learned through experience and reinforced every time something goes wrong.
Getting Real About Solubility
N-Sulfopropylpyridinium trifluoroacetate doesn’t come up much in casual conversation, but for research labs and chemical engineers, it plays an important role. I remember a project troubleshooting a stubborn protein purification step that never worked if the additive didn’t dissolve properly. Your solvent choice can make or break the workflow, and that becomes clear with a molecule like this.
Reports and direct experience back up the same core result: N-sulfopropylpyridinium trifluoroacetate breaks down best in polar solvents. Water and methanol do the trick, while nonpolar solvents like hexane leave you frustrated with cloudy suspensions. My old lab bluntly called it “salt logic”—polar molecules dive into polar solutions. Researchers working with ionic liquids or charged compounds relate, since water's high dielectric constant makes it a go-to for dissolving these kinds of molecules. Quick-dissolve traits save time and prevent batch-to-batch inconsistency.
Compatibility in Real Lab Life
Anyone running liquid chromatography, mass spec assays, or ion-exchange knows compatibility isn’t a bonus—it’s vital. The pyridinium head on this chemical interacts well with a wide range of cations and anions, helping stabilization in solution. That matters most when mixing buffers or running multi-step syntheses, where even a small incompatibility can ruin a dataset or blow a whole week.
I once saw a student use this additive to modify a separation protocol for peptides. It stood up to acidic and mildly basic pH, surviving routine acetonitrile gradients and working well with common protein stabilizers. Not all additives can pull that off. The trifluoroacetate group also brings electric charge and offers some shielding against background ions, which limits unwanted interactions in high-sensitivity detection. According to literature published in Analytical Chemistry and several online protocols, it doesn’t mess with most biologically relevant cations or clog up columns with precipitates.
Challenges and Solutions
There’s no denying things get trickier in high-concentration salt solutions. I’ve seen precipitation pop up when stock solutions get too saturated, especially if mixed with divalent cations like calcium or magnesium. Keeping concentration reasonable and running small pilot experiments helps sidestep this. For those scaling up or working in new systems, gradually increasing test concentrations saves time and cuts down on bench waste.
Another tricky point—storage. This isn’t something you want lying open near strong bases or acids. Leaving the bottle in a humid corner or open to air can change the salt’s character and affect later use. Dry vials kept at stable temps will protect shelf life and reliability.
Practical Tips for the Lab
In practical terms, dissolving N-sulfopropylpyridinium trifluoroacetate in water or low concentrations of common alcohols works fast. Mixing should stick to vortexing or gentle stirring; excessive shaking or heating brings more trouble than it solves.
Taking a step back, I’ve seen too many labs skip checking for chemical compatibility before jumping into experiments. Investing time in solubility tests never feels wasted compared to the headaches caused by an incompatible buffer or a surprise precipitate. Collaborative platforms like PubChem and Sigma-Aldrich continue updating compatibility and handling data as real users share new findings, which boosts reliability and keeps expensive mishaps rare.