Commentary: Nonyl 8-Bromooctanoate—Examining Its Background, Science, and Impact
Historical Development
Nonyl 8-Bromooctanoate didn’t pop up overnight. Its origins tie back to the late twentieth century, as chemists started exploring new brominated compounds for industrial and research uses. Before this, octanoate esters and brominated side chains showed promise in laboratories for surfactant and specialized synthesis. Then, companies and research teams began tinkering with nonyl groups linked to bromooctanoate, often in search of stability and performance tweaks for advanced industrial processes. Each tweak made a difference: one modification made separation easier, another added thermal durability. After a while, this led to what process engineers and chemists now recognize as a workhorse reagent, especially as techniques for bromine introduction improved and labs gained access to cleaner, safer reactants.
Product Overview
Nonyl 8-Bromooctanoate catches the eye of chemical manufacturers for more than its mouthful of a name. It brings together the fatty acid backbone found in octanoates with a bulky nonyl group and a bromine atom in just the right spot. It's the kind of compound you see listed in catalogs with product numbers and purity grades, not something that winds up in a household cleaner or medicine bottle at the pharmacy. Instead, companies stock it as an intermediate or reagent, ready to plug into other recipes—especially specialty surfactants, functional coatings, and research samples. Its appeal comes from that combination, letting it avoid the pitfalls that plague simpler esters or plain brominated hydrocarbons.
Physical & Chemical Properties
Ask anyone who’s handled Nonyl 8-Bromooctanoate about its look and feel, and they’ll mention its oily nature at room temperature. It's often a pale yellow to colorless liquid, heavier than water because of its bromine content. The strong molecular weight shows up on the scale, while that long carbon chain prevents it from evaporating too fast. Clients expect decent solubility in organic solvents like chloroform or toluene, but water and polar solvents don’t do much with it. The bromine makes the compound reactive, especially when heated with strong nucleophiles or in the presence of light, but it resists breaking down on the shelf. This matters in warehouses where turnover might take a while, and stability means safer storage.
Technical Specifications & Labeling
Regulatory teams label Nonyl 8-Bromooctanoate with CAS numbers, storage codes, and sometimes barcodes, since tracking hazardous intermediates draws regulatory scrutiny. Labels spell out purity percentages—above 97% counts as high grade for industry. Labels and Safety Data Sheets also warn about the chemical’s bromine content, boiling points, and storage temperature. Some suppliers toss in extra information, like recommended shelf life, container material (glass over plastic), and expiration dates. From packing to shipping, regulatory compliance teams keep a close eye, since a missed number or missing warning could mean fines.
Preparation Method
Most synthesis uses a two-step route, adding a nonyl group first, then introducing bromine at the octanoate chain’s eighth carbon. Chemists often prefer the esterification of 8-bromooctanoic acid with nonyl alcohol using acid catalysts, sometimes with molecular sieves or azeotropic distillation to pull off water and push the reaction further. Modern labs trend toward solvent-free or low-solvent methods, both for safety and cost control, though batch reactors still rely on conventional solvents when chasing high yields. The main synthetic headache is controlling regioselectivity so bromine lands only on carbon number eight. It takes close monitoring, with columns or distillation at the end to clean up byproducts.
Chemical Reactions & Modifications
Nonyl 8-Bromooctanoate acts as a versatile stepping-stone in labs hunting for more complex molecules. That bromine means nucleophilic substitution comes easy. Drop in a strong base or a nucleophile like an amine, and the bromine leaves, replaced by another functional group in a reaction that’s easy to tune. Maybe you want a new amide or ether—chemists often lean on classic SN2 mechanisms for clean transitions. Under the right light or in the company of catalysts, radical reactions can also break that C–Br bond, opening paths to cross-coupling or chain extension. Each tweak brings a chance for new physical behaviors or chemical properties.
Synonyms & Product Names
Browsing supplier catalogs or research papers, Nonyl 8-Bromooctanoate pops up under a string of alternative names. Names like "Nonyl bromooctanoate," "8-Bromooctanoic acid nonyl ester," and abbreviated forms using its CAS digits all signify the same molecule. A quick scan shows these aliases help scientists track older studies, spot fraudulent or off-brand sources, or clarify details in translation between supplier lists. There’s also a spread of product codes between major suppliers, usually tied to stock-keeping units and batch records. For someone checking legal or patent boundaries, it’s important to watch every name and cross-reference data before buying or using in commercial-scale runs.
Safety & Operational Standards
Lab safety officers pay extra attention to chemicals with bromine and medium-chain fatty esters. Nonyl 8-Bromooctanoate fits that bill. Skin, eye, and respiratory protections matter, since accidental spills or vapors, especially under heating, can bring irritation or worse. Fume hoods and gloves remain the norm, not the exception, during synthesis or handling. If stored long-term, it needs tight seals and cool, dark locations to reduce unwanted reactions. Safety Data Sheets, updated with each batch, give clear instructions on fire, spill, and exposure responses. Some operations go as far as keeping spill kits and neutralizers nearby, remembering past incidents when lax practices led to ruined batches or regulatory violations.
Application Area
Industries touch Nonyl 8-Bromooctanoate mostly in specialty chemical production and research labs cranking out bespoke intermediates. It serves as a building block in surfactant synthesis, letting chemists tune hydrophobic and hydrophilic balance on demand. For high-end lubricants or additives, this molecule’s stability and reactivity make it a best-fit link. Some polymer chemists work it in for side chains, especially in projects targeting surface properties or unique plasticizers. Don’t expect it in pharmaceuticals yet—drug chemists remain wary of the bromine, and regulators haven’t cleared it for oral or topical use. Still, the industrial research push sees this compound show up in coatings, water repellents, and advanced material science departments.
Research & Development
Modern R&D leans hard into brominated esters like Nonyl 8-Bromooctanoate, revisiting synthesis for efficiency, safety, and environmental performance. Green chemistry initiatives encourage switching over to fewer solvents, recyclable catalysts, and milder temperatures. Research teams keep publishing about alternative feedstocks to dodge petroleum dependencies, looking to renewable fatty acids and alcohols sourced from plant oils. Some labs pair this molecule with trendy analytics—spectrometry, chromatography—probing for trace impurities and new reaction routes. Collaborations show up between startups and universities, each team probing new niches: functional coatings, smart adhesives, and stimuli-responsive materials. Each project faces questions on scalability; laboratory methods often look different in a warehouse reactor, challenging both process and safety teams.
Toxicity Research
Safety studies focus on immediate and chronic toxicity, given brominated compounds’ checkered past in ecosystem and health impacts. Toxicity profiles for Nonyl 8-Bromooctanoate track both in vitro (cell-based) and in vivo (animal) effects, so far indicating moderate acute toxicity at high exposure, mostly skin and respiratory irritation, but less reproductive or carcinogenic risk compared to shorter-chain analogues. Environmental models still worry about breakdown products, since some brominated organics hang around in soil and water. Disposal plans now require more paperwork: controlled incineration, or recovery in certified disposal facilities. Regulatory agencies keep a watchful eye for new animal or environmental impact data, and many chemical safety boards ask labs and factories to maintain logs for long-term exposure cases. Anyone scaling this compound for industry often has to weigh the increased reporting burden alongside process benefits.
Future Prospects
As demand for fine chemicals and tailored surfactants grows, so does the outlook for intermediates like Nonyl 8-Bromooctanoate. Firms track its path from lab to market, betting on new polymer blends, standout lubricants, and niche adhesives—all built on customization at the molecular level. Some hope advances in catalysis and green chemistry will cut production costs and shrink environmental footprints, while others push for digital modeling to speed up development cycles. Regulatory trends challenge manufacturers to invest in safety, tracking, and eco-impact analysis. The future likely belongs to companies balancing process innovation with visible stewardship, staying transparent about supply chains and product fate. My own experience in industrial labs reminds me: compounds once seen as mere reagents can turn into cornerstone products if teams pay attention to the full chemistry, from the flask to the factory floor.
What Makes Nonyl 8-Bromooctanoate Worth Talking About?
Nonyl 8-Bromooctanoate rarely shows up in headlines, but this chemical quietly plays a role in shaping daily products that most folks take for granted. Its distinct molecular setup appeals to those working in fields where function and reliability matter more than flashy marketing. This appeal lies in stability, resistance to harsh environments, and a record of delivering consistent results across very different applications.
Breaking Down Key Industrial Uses
Big players in the plastics sector often turn to Nonyl 8-Bromooctanoate as a reliable plasticizer. A plasticizer gives polymers the needed softness and flexibility that hard plastic just can’t offer by itself. Think about wires snaking behind your TV or headphones that don’t snap after a week. These products require the right blend of toughness and bend—qualities that Nonyl 8-Bromooctanoate brings to the table.
Paints, sealants, and coatings also form a core market for this compound. It provides sustained resistance against water, chemicals, and even sunlight, which is crucial for surfaces exposed to the outdoors or rough conditions. This unique resistance extends product life and helps prevent costly repairs that come when coatings fail. From construction sites to car parts, workers rely on that added layer of protection to deliver performance that holds up over the long haul.
Unexpected Roles in Lubricants and Industrial Fluids
Factories keep machines running with help from specialty lubricants. Nonyl 8-Bromooctanoate slips into these formulas to keep parts gliding without overheating or locking up. Modern manufacturing lines push equipment to the limit. High stress and continuous friction mean that a lubricant must stand up, job after job. Formulators reach for chemicals that have a track record of protecting gears and bearings, saving headaches for engineers and plant managers.
Specialty fluids in electronics and advanced engineering also benefit from the chemical’s adaptability. It doesn’t break down when exposed to the challenges that industrial environments throw at it, from high heat to exposure to various substances. This dependability keeps processes running smoothly, which many workers know is never guaranteed when cutting corners on material quality.
The Drive Toward Safer, More Sustainable Chemistry
People are growing more concerned about what goes into everyday products. In my experience covering the chemical industry, regulatory agencies and consumer groups watch chemical additives closely. Nonyl 8-Bromooctanoate, like many specialty additives, faces pressure from groups pushing for safer, greener alternatives. Researchers have responded by testing for environmental impacts and exploring bio-based options, but transitions always take time in industries built around consistency and strict safety demands.
Better labeling, transparency in sourcing, and rigorous safety reviews help companies reassure the public about these chemical choices. End-users—whether it’s a parent picking a paint or a company designing consumer electronics—want straight answers about risk. Long-term, the industry may pivot to new molecules that perform just as well but satisfy regulators and environmental groups. But for now, Nonyl 8-Bromooctanoate stays in demand, thanks to its proven record and the challenges of developing drop-in replacements.
Where Value and Vigilance Meet
Nonyl 8-Bromooctanoate may never become a household name, yet the things it makes possible shape the working world for millions. Keeping an eye on its role and impact connects industries, end users, and regulatory bodies in a bigger conversation about safety, performance, and progress. As someone who’s spent years following the evolution of specialty chemicals, I see the delicate balance between proven tradition and the push for safer innovation as a test every material in this class faces sooner or later.
Breaking Down the Name
Chemical names can sound a bit intimidating at first glance, but they reveal a lot about what’s inside. Nonyl 8-bromooctanoate packs several clues in its name. The “nonyl” part refers to a nine-carbon straight chain, like a tail attached to the oxygen in the molecule. The “8-bromooctanoate” tells you there’s an eight-carbon fatty acid backbone that includes a bromine atom on the eighth carbon. Put together, nonyl 8-bromooctanoate forms an ester, a result of the reaction between nonanol (nine-carbon alcohol) and 8-bromooctanoic acid.
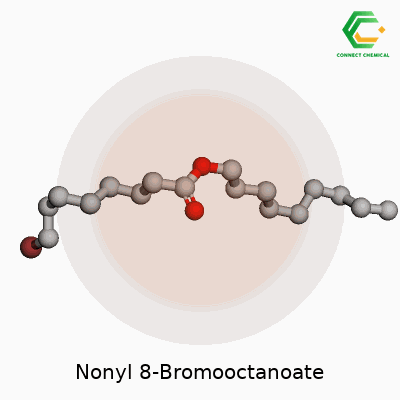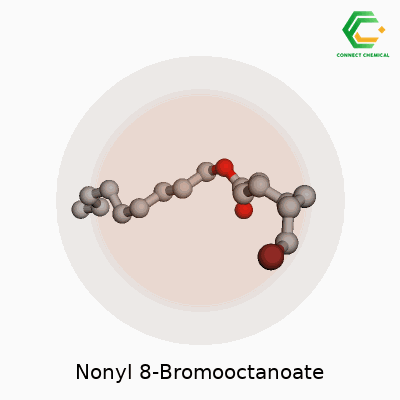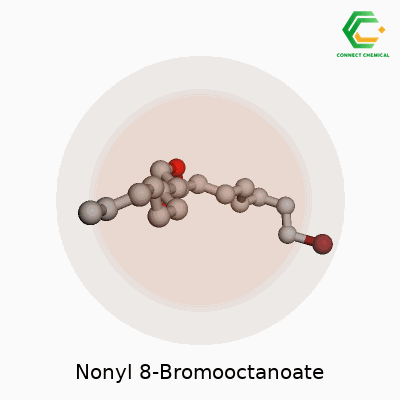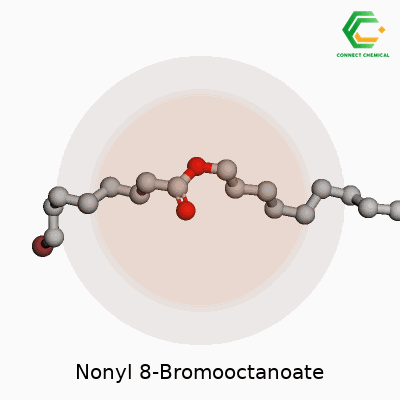
Molecular Formula and Structure
To work out the molecular formula, consider what each part contributes. Nonanol is C9H19OH. 8-bromooctanoic acid is C8H15BrO2. During esterification, a molecule of water comes off, connecting the oxygen from the alcohol and the acid’s carbonyl carbon. The resulting molecular formula becomes C17H33BrO2. This includes nine carbons from the nonyl group and eight from octanoate, along with a single bromine atom.
The structural backbone tells a story of long carbon chains, which is typical for esters used in specialty chemical applications such as surfactants or plasticizers. In practice, the large hydrophobic sections of these molecules allow for unique properties: think of oil spills and how surfactants help break up slicks. The placement of the bromine atom at carbon-8 makes the molecule more reactive than plain esters, introducing sites for further modification or breakdown in the environment.
Why Chemical Structure Matters
The arrangement of atoms in molecules like nonyl 8-bromooctanoate controls how they behave in water, soil, and even in living organisms. Having a bulky nonyl group on one end and a brominated octanoate on the other sets this compound apart from typical fatty acid esters. Bromine’s presence often signals potential persistence in the environment, sometimes even raising concerns over bioaccumulation and safety for aquatic life.
Industry relies heavily on esters for their lubricating and emulsifying properties. The appeal of branching out with nonyl chains and brominated fatty acids lies in tuning melting points, solubility, and breakdown rates. This kind of customization often helps in designing better detergents or specialty oils, and it’s not just limited to laboratory circles; these tweaks frequently end up affecting everyday products.
Concerns and Next Steps
Brominated chemicals prompt stronger scrutiny these days. Evidence links some brominated organics with disruptions in wildlife and potential health questions for humans. It’s important to weigh functionality against safety. I’ve come across research that urges alternative pathways—chemists have started using greener catalysts or finding new ways to introduce reactive groups without relying on halogens like bromine.
There’s a push within the scientific community to explore biodegradable esters and avoid persistent halogenated compounds. Regulatory guidelines across Europe and North America keep a close watch on any new brominated chemical entering the market. One potential solution: invest in robust toxicity studies early, and lean on natural sources or benign modification methods whenever possible. Companies that integrate these values into their development process often avoid future headaches and align with sustainability goals.
Understanding nonyl 8-bromooctanoate’s structure and formula doesn't just satisfy academic curiosity—it plays a big role in tackling real-world environmental and safety issues. Chemistry doesn’t live in isolation; every new compound carries impacts far beyond the flask.
Understanding What’s at Stake
Dealing with chemicals like Nonyl 8-Bromooctanoate means facing real risks if storage and handling slip through the cracks. I’ve spent over a decade in industrial labs, and stories of close calls always come back to basic safety steps that got skipped. This compound, used in specialty syntheses and surfactant development, won’t forgive shortcuts. A lack of respect for chemical safety can leave people dealing with fires, toxic vapors, or even injury. Real people, real workspaces—there’s too much on the line to wing it.
Keep Compatibility Front of Mind
Nonyl 8-Bromooctanoate won’t play nice with strong acids, bases, or oxidizers. Pushing containers into the same storage bay as reactive substances feels tempting when space runs tight, but it only takes one leaky cap or careless transfer to set off a chain reaction. Every reputable manufacturer includes a safety data sheet (SDS) that calls out common hazards. In my time, I’ve seen folks ignore those details and pay the price—burnt rags, smoking trash cans, or a cloud of bad news wafting through a poorly ventilated room.
Temperature and Light Make a Difference
This compound doesn’t like heat, humidity, or bright light. I once watched a batch degrade because a storeroom relied on a sunny window and the air conditioner never kicked in. Result? A sticky, discolored mess and a pile of wasted money. Cool, dry, and dark places keep chemical reactions to a bare minimum. Insist on a climate-controlled space and you’re already cutting the biggest risks off at the knees.
Sealed Containers and Smart Labeling
Tight-sealing containers never seem glamorous until someone forgets to close one and vapor builds up, or a spill spreads across the bench. This stuff isn’t water—its volatility and toxicity climb the ladder quickly. Manufacturers usually ship Nonyl 8-Bromooctanoate in HDPE or glass bottles with proper caps. I label every bottle with clear, waterproof ink. Fuzzy handwriting or faded labels have caused more misunderstandings than I dare count, especially during emergencies.
Personal Protection: Not Optional
Gloves, goggles, and lab coats stand between you and a trip to the hospital. The liquid can affect skin and eyes. I’ve seen new technicians treat PPE like an afterthought, peeling gloves mid-task or adjusting their goggles with wet hands. The consequences show up fast—burns, rashes, and, at worst, chemical inhalation. Those accidents haunt everyone in the building, not just the person who let down their guard.
Preparedness Saves the Day
Regular drill runs beat reading warnings after the fact. Everyone working near Nonyl 8-Bromooctanoate needs to know where the nearest safety shower, eyewash station, and spill kit sit. I’ve witnessed trained teams handle spill scenarios calmly while chaos erupted in less prepared shops. Proper ventilation and air filtration—especially in enclosed labs—mean the difference between a minor cleanup and a company audit.
Building a Safety Culture
Mistakes stack up where people treat dangerous chemicals like office supplies. Every supervisor, every tech, every supplier has a role. Staying up to date with training, reading the SDS, and checking container seals isn’t just bureaucracy—it’s how everyone gets home safe at the end of a shift. Ignoring small steps leads to big costs, for people and for business. Respecting these procedures reflects experience, care, and a direct investment in the well-being of everyone around the bench.
Chemical Curiosity Grows
Questions have started swirling about nonyl 8-bromooctanoate, a chemical with a mouthful of a name but little in the way of mainstream attention. Chemical safety rarely makes the headlines. Still, it hits home in labs, factories, and any place people worry about what touches their skin or food. If a new or obscure compound surfaces, even one not widely used, it draws a certain audience: folks who value transparency and want straight answers.
What We Know So Far
Taking a walk through the available literature turns up a silence that almost shouts. Nonyl 8-bromooctanoate floats below the radar in major safety databases. No entries in the U.S. EPA’s chemical hazards spotlight. No peer-reviewed toxicology studies in major journals. Even the European Chemicals Agency, known for flagging chemical hazards early, has yet to weigh in publicly. This absence invites both relief and worry. It could mean no one has flagged big problems, or just that no one's looked close enough.
Lack of published toxicity data rings alarm bells for those of us who came up in research labs. Many scientists remember hits and misses with chemicals thought harmless at first glance. Subtle effects can hide in formulas and obscure structures, and health impacts pile up long after first contact. Early in my career, I watched as substances, once accepted, slowly revealed their health risks—sometimes long after workers already felt the consequences.
What Happens Without Transparency
Anyone who's followed chemical safety knows silence rarely means a guarantee of safety. Chemicals showing up in research, product manufacturing, or industrial processes deserve scrutiny. Questions about inhalation risks, skin contact, and long-term exposure matter, even for compounds not listed as officially hazardous—especially so, considering we used to toss around solvents and cleaning agents with little thought, only to chase after health disorders years later.
Practical experience reminds me that workplace safety rules grow out of real mistakes. Every time a compound stays off the radar, workers and consumers run the risk of becoming accidental guinea pigs. If manufacturers or researchers start using nonyl 8-bromooctanoate on a wider scale, a public record and transparent reporting matter more than ever. Relying on a lack of warning labels or missing hazard icons leaves too much to chance.
How We Could Do Better
Better chemical oversight is possible with upfront, independent testing. Chemicals, especially those with long, unfamiliar names, benefit from mandatory baseline toxicity studies before any large-scale exposure. The industry would serve everyone better by supporting open data initiatives, so anyone—from healthcare professionals to university students—can see what’s known, and what’s not, about emerging compounds.
Real solutions depend on trust and openness. If a product relies on nonyl 8-bromooctanoate, companies should support routine third-party reviews, fund research into inhalation and skin exposure effects, and keep the public looped in. Regulators play a part, but the loudest call for chemical testing and data transparency usually comes from those who wear the gloves, pour the flasks, and keep businesses running. Until more information comes to light, treating new substances with healthy skepticism and responsible routines is the safest bet.
Real-World Demands in Chemical Packaging
Nonyl 8-Bromooctanoate pops up in laboratory catalogs, chemical warehouses, and on spreadsheets for process engineers. Anyone who’s had to store, transport, or even dispose of specialty chemicals knows packaging carries as much weight as purity or regulatory status. It’s not about the label looking sharp, but about whether you can pour, recalibrate, or ship the stuff safely and cost-effectively.
What’s on the Market? Actual Formats Available for Ordering
Most chemical suppliers offer Nonyl 8-Bromooctanoate in either small glass bottles, HDPE jugs, or metal drums. I’ve ordered up enough specialty chemicals to realize that most research labs pick up 100-gram or 500-gram amber vials. These often come with PTFE-lined caps, which prevent leaks and stop unwanted reactions from creeping in. Medium-scale purchasers—think pilot plants or universities running repetitive tests—usually step up to 1 kg or 2.5 kg jugs. These jugs offer a break between tiny research sizes and production drums, and the HDPE material shrugs off most bumps and spills on busy benches.
Full-scale production buyers often want 25 kg or 50 kg drums, usually made out of steel lined with epoxy resin. A regular chemical warehouse manager will back me up: the real reason for this choice isn’t just volume. It’s about stacking strength, forklift access, and tighter seals during transport. I’ve watched more than a few drums travel halfway across the world, and unless the packaging holds up through customs, leaking chemicals become an urgent problem for everyone involved.
Why Packaging Standards Matter
Most shipments of Nonyl 8-Bromooctanoate stay under strict chemical safety rules. Countries might set UN specifications, or demand packaging that’s been pressure-tested and labeled with clear hazard symbols. I’ve managed chemical storerooms packed with incompatible reagents and solvents, and the labeling and compatibility information on the drum saves real headaches. Not every chemical needs a vacuum-sealed liner, but with some esters and halides, moisture or air exposure means ruined stock.
Scale Drives Choice—and Cost
Suppliers often push toward economy of scale; bulk pricing always draws big buyers, yet waste becomes a concern if you don’t use the chemical soon enough. I had a client order a single 50 kg drum, thinking they’d use it up within two months. By the end of the year, half the material had gone bad. The lesson? Size your packaging not just for price breaks, but for actual consumption and stability. Local resellers will sometimes split up drums for smaller industries or research groups, which helps maintain fresh inventory.
Sustainability, Safety, and Future Trends
The chemical industry faces more scrutiny about plastic waste and hazardous byproducts. Major producers have started to offer bulk packaging in reusable or recyclable formats—steel returnable drums, for instance—or customer recycling programs for emptied containers. End users need concrete information about material compatibility and return policies. For years, labs tossed empty containers as hazardous waste. Now, some companies help streamline returns, cutting down both hazardous waste costs and environmental impact.
Pushing for a Smarter System
Supplier transparency stands out as a missing link. Customers shouldn’t have to call for a safety sheet or detailed storage guideline; digital catalogs could offer better visibility up front. I’d welcome a world where you can choose packaging not just by size, but by shelf life, recyclability, and delivery footprint. Until then, checking labeling, thinking through actual usage rates, and partnering with reliable suppliers makes all the difference.