Octadecylbis(2-Hydroxyethyl)Methylammonium Chloride: A Deep Look
Historical Development
Chemistry has always sought new compounds for industrial and scientific advancement. Octadecylbis(2-hydroxyethyl)methylammonium chloride owes its existence to the rising need for surfactants and specialty chemicals across the 20th century. Laboratories started focusing on quaternary ammonium compounds as scientists found these molecules delivered strong antimicrobial effects and demonstrated impressive surface activity. Early patents in the mid-1900s set out methods for combining long-chain alkyl groups with hydroxyethyl and methyl functionalities. In the decades that followed, this specific type earned a steady place in cleaning agents and textiles due to performance under tough processing conditions and physical stability, qualities that many early surfactants struggled to deliver. When I first started working with specialty chemicals in the lab, colleagues often spoke about breakthroughs in process chemistry for quaternary ammonium compounds, as the demand for more robust surfactants, fungicides, and emulsifiers kept growing with industry expansion.
Product Overview
Octadecylbis(2-hydroxyethyl)methylammonium chloride appears as a waxy solid or a viscous, crystalline substance, often sold as powder or concentrated aqueous solution. This compound belongs to the family of cationic surfactants—a category valued for its antimicrobial punch and ability to modify surfaces. Its impressive hydrophobic tail (the octadecyl group) interacts strongly with lipid membranes, which matters in disinfection and cleaning. Products using this compound turn up in everyday scenarios, from antimicrobials for hospital use to softeners and antistatic additives in the textile industry. What separates it is a blend of cleaning power and a softness imparted to surfaces, whether that’s skin, fabric, or industrial equipment.
Physical & Chemical Properties
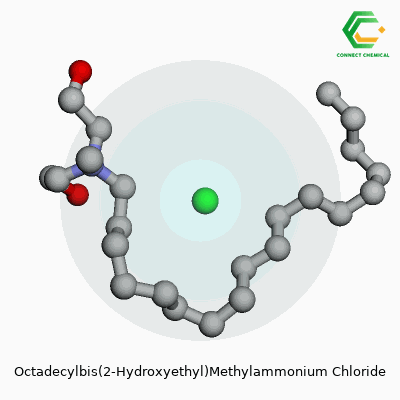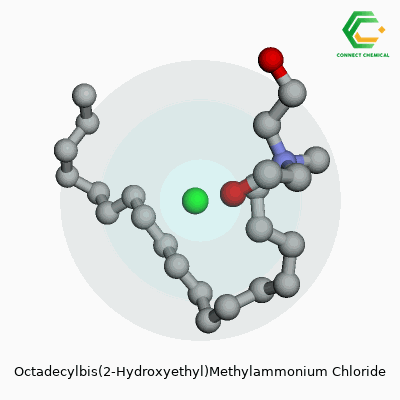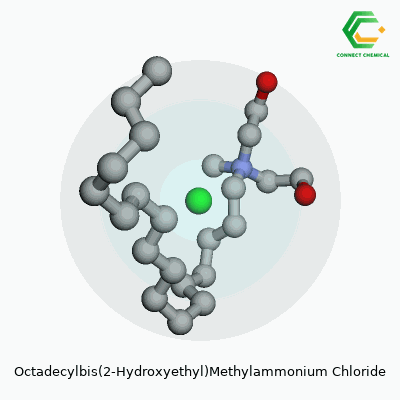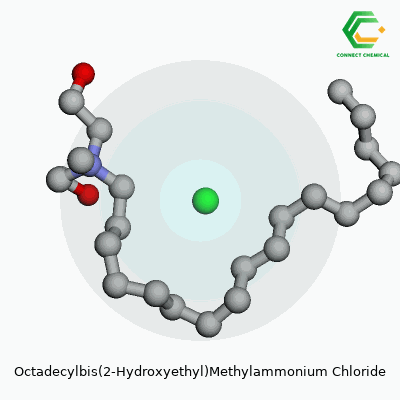
With a chemical formula of C23H50ClNO2, this molecule carries a long hydrocarbon chain that makes it semi-solid at room temperature. I’ve noticed its strong performance in water, thanks to its good solubility—the chloride anion helps maintain this feature in polar environments. Molecular weight falls in the mid-400s, giving some heft compared to more basic surfactants. Its melting point sits around 60–80°C, and the presence of secondary alcohol groups means it blends or emulsifies fat, grease, and oil quite efficiently. In basic lab work, it shows both surface activity and cationic charge, making it responsive to mixing with negatively charged dyes, fibers, and proteins.
Technical Specifications & Labeling
Commercial bottles show purity grades above 97%, with moisture content tightly controlled below 1%. Labels often highlight pH range stability (between 4 and 9), along with shelf life which routinely pushes two years if kept airtight and cool. Technical sheets include recommended handling temperatures, flash point (typically above 150°C), and storage cautions related to heat and direct sunlight. Regulatory labels from REACH and GHS appear, with standard warnings about eye and skin contact. Transport and shipment information detail CAS numbers and proper UN codes given its status as a non-flammable, low-toxicity surfactant.
Preparation Method
Manufacture relies on the alkylation of methylbis(2-hydroxyethyl)amine with 1-chlorooctadecane in an organic solvent. I remember our plant used large stirred reactors, with temperature profiles carefully monitored to avoid side reactions and decomposition. The reaction proceeds well under reflux conditions, with quaternization taking several hours. Purification—vital for downstream consistency—involves repeated washing with water and organic solvents followed by vacuum drying. In our facility, we always focused on controlling input reactant quality; even minor impurities would reduce yield or generate off-color final product, so source control and sampling featured heavily in QC routines.
Chemical Reactions & Modifications
Chemically, octadecylbis(2-hydroxyethyl)methylammonium chloride holds up well under moderate pH and temperature. The hydroxyethyl arms can produce esters with carboxylic acids, allowing for the introduction of scent molecules or fabric protectants. In practice, I watched our R&D team tailor surface-activity or antimicrobial properties by swapping methyl for ethyl groups or altering alkyl chain length, tuning solubility profiles for whichever process needed them. Chemical modifications sometimes include polymerizing the hydroxyethyl moieties to produce specialty resins or to immobilize the active molecule on filter media.
Synonyms & Product Names
Suppliers rarely stick to one name. You’ll find labels reading N,N-bis(2-hydroxyethyl)-N-methyl-octadecylammonium chloride, or abbreviations like ODHEMAC or behenyl hydroxyethyl methyl ammonium chloride. Specific product names depend on grade and target industry, sometimes referencing “quat-amine” or “hydroxyethylated quaternary ammonium salt.” Our purchasing team kept a detailed database to track codes from each supplier since confusion over synonyms could cause errors in order processing or regulatory document filing.
Safety & Operational Standards
Routine handling calls for gloves and eye protection. Contact with eyes or mucous membranes can cause mild to moderate irritation—less pronounced than harsher surfactants, but still present. Safety kits in our lab included eyewash stations and proper air extraction, since inhaling powder or concentrated aerosols can irritate airways. Storage takes place in tightly sealed drums within cool, shaded warehouses. Spill response guidelines focus on dilution and collection, not neutralization, owing to the compound’s cationic and semi-biological nature. Standards from OSHA and CEFIC set thresholds for occupational exposure, surface contamination, and permissible residue in finished goods. Training for new staff covers GHS labeling and first-responder advice in case of a leak or mix-up, even though long-term toxicity is considered low.
Application Area
Market demand for this compound stretches across household disinfectants, hair conditioners, textile rinses, antistatic coatings, and some specialty lubricants. I’ve worked alongside engineers who valued it for reducing friction in spinning threads, while others mixed it into sanitizing sprays for food processing lines. The antimicrobial activity disrupts biofilms and stymies the growth of mold and bacteria, contributing to health standards in hospital and nursing home environments. Its surface conditioning reduces the charge build-up in synthetic fibers, making it a staple in carpet finishing plants.
Research & Development
Academic and industrial research focuses on improving biodegradability and reducing aquatic toxicity. Over the past decade, teams have tested derivatives with shorter hydrocarbon tails to check if the cleaning power stays strong but environmental persistence drops. I took part in efforts to blend the quaternary ammonium backbone with renewable feedstocks, looking for balance between ecological impact and the product’s stalwart performance. Peer-reviewed journals fill up with studies dissecting molecular pathways, hoping to preserve functional benefits while addressing regulatory concerns. Partnerships between chemical companies and universities have grown common, since tight environmental rules drive demand for green chemistry innovations.
Toxicity Research
Several studies show low acute toxicity in mammals. Ingestion can cause gastrointestinal irritation, but systemic poisoning requires high doses well above occupational exposure. Environmental impact remains, as concentrations in wastewater treatment systems can linger and sometimes impact freshwater organisms such as Daphnia magna and algae. In controlled conditions, skin contact rarely triggers allergies. Longitudinal epidemiological studies remain scarce, a gap usually flagged by public health experts. Regulatory groups watch out for cumulative effects, yet current evaluations by EPA, ECHA, and similar bodies put it in a class requiring standard chemical hygiene—not panic buttons. Extensive wastewater treatment and biodegradation tests continue, especially as stricter EU and North American discharge regulations roll out.
Future Prospects
Demand for safe, biodegradable, non-irritant surfactants keeps pushing chemists to tweak established formulas. Octadecylbis(2-hydroxyethyl)methylammonium chloride will carry on as a workhorse for disinfecting and softening—unless a dramatic leap in biotechnological alternatives disrupts the sector. I expect future R&D to zero in on upcycling agricultural byproducts as feedstock for its synthesis, and also see digital process controls tightening quality and energy management on manufacturing floors. Stricter rules around environmental fate and microbial resistance will probably shift usage toward specialty applications where performance can’t be traded for cheaper, less effective alternatives. Product stewardship programs will grow, improving traceability from source to destination, and tightening the feedback loop between health, safety, and industrial chemistry.
What Is It?
Octadecylbis(2-hydroxyethyl)methylammonium chloride has the kind of name that brings chemistry class nostalgia rushing back. For many, this mouthful doesn’t sound very familiar, but for folks working in industries from textiles to cleaning products, it plays a bigger role than you’d expect.
Everyday Uses You Don’t Notice
My years working in product development exposed me to a surprising variety of surfactants and antistatic agents. This compound shows up on the ingredient list of fabric softeners for good reason. It conditions fibers and keeps static cling to a minimum. Pulling those laundry loads apart on a dry winter day, you’ll notice how much easier it feels with some chemistry doing invisible work in the background.
Beyond softening clothes, manufacturers add it to hair conditioners, creams, and some lotions. It doesn’t just improve texture — the molecule bonds with surfaces, smoothing down frayed cuticles or rough spots on fabrics. I’ve seen test results: towels treated with this compound last through repeated wash cycles without getting scratchy or losing absorbency. Consumer feedback often mentions a “fresh” or “clean” feel, and this ingredient deserves credit there.
Industrial and Environmental Footprint
Paper processing plants and textile mills also lean on this chemical to keep machinery moving smoothly. In the paper business, it’s used to make sheets less prone to static and more agreeable for ink. This helps prevent smudges and misprints, something I’ve seen frustrate many a print shop manager. It pops up in anti-fogging agents for glass and plastics, too. Ever wondered why a mirror or car window gets less foggy after cleaning with certain sprays? Look at the label — there’s a fair chance this ammonium compound appears near the bottom.
All this usefulness brings a price. Surfactants don’t always break down quickly after being washed down the drain. Some studies have linked quaternary ammonium compounds to water toxicity issues in aquatic life when treatment plants fail to filter them out. My time in industrial compliance taught me how much stress companies feel keeping up with shifting waste standards and public concern. European regulators, for example, keep tightening permissible discharge levels of these compounds, pushing companies to rethink formulations.
Finding the Balance: Safer Choices
Industry doesn’t stand still. Biodegradable alternatives are taking hold in some laundry and cleaning sectors. Brands advertising “green chemistry” often highlight their move away from traditional quats like this one. Some manufacturers now blend in enzymes or plant-based surfactants that cause less impact downstream. At conferences, I’ve noticed a lot more research posters studying life-cycle analysis and looking for substances that deliver performance with less environmental baggage.
Consumers help drive change when they check labels and ask tough questions. Plenty of customers now want to know what makes their towels soft or their hair smooth. Public pressure has nudged brands to test and publish safety data transparently. This feedback loop between science, industry, and the people who buy the final product keeps reforms moving. Not every ingredient with a complicated name deserves suspicion, but every one deserves a closer look to weigh its benefits and risks.
Understanding the Real Risks on the Shop Floor
Octadecylbis(2-hydroxyethyl)methylammonium chloride sounds intimidating, and seeing it on a label often stops people in their tracks. It gets used in antistatic agents, textile conditioning, and as a component in fabric softeners. Sometimes, it lands in technical settings — industrial laundries or plastics manufacturing. I’ve worked around specialty chemicals for years, balancing protective measures and the daily grind. Knowing more about this chemical’s safety can help people in labs and factories avoid risky shortcuts.
Known Hazards: Fact over Fear
Every chemical on the job carries specific hazards. The first place I check is the safety data sheet (SDS). This one usually lists skin and eye irritation as top concerns. Accidentally splashing it on your skin causes painful redness and sometimes even burns. Eyes are even more sensitive. If you ever end up with a drop of it in your eye, flushing with water won’t feel comfortable. Prolonged contact, especially without gloves, almost guarantees trouble.
Respecting personal protective equipment isn’t just a box to check. Standard practice means wearing nitrile gloves, safety glasses, and sometimes even aprons if an operation involves lots of splashing. Ventilation plays a role, too. I’ve seen tight corners and closed rooms set off bigger incidents than open, breezy spaces. Inhaling the spray mist or dust poses risks — coughing and throat irritation happen fast. No one wants to rush to medical for a breathing issue that a simple fume hood could have prevented.
Long-Term Effects: What Science Says
There’s always concern about chronic exposure. For this compound, parameters look a little better compared to harsh solvents or cancer-linked chemicals. I checked recent toxicology reviews — no solid evidence points to it causing cancer or reproductive harm in normal industrial use. Environmental impact remains a discussion point since quats like this don’t break down as easily as surfactants found in soap. Companies watch their wastewater and disposal methods to cut down on pollution upstream and downstream.
Practical Steps for Handling
Every veteran I talk to agrees: cutting corners to save a minute rarely pays off. Simple steps become habits after a while. Start with reading the container label each time, even if the packaging looks the same as old stock. Gloves should fit tightly with no holes. In case of a spill, grab the spill kit — at least a handful of absorbent pads and neutralizing powder if available. Dispose of cleanup material per local regulation; toss it in a general trash can, and you risk contaminating groundwater.
Training walks matter. I remember one time an apprentice got a bit on their hand, shrugged it off, and ten minutes later needed first aid. People sometimes think “it’s just fabric softener stuff”— but high-concentration forms don’t play by consumer product rules. Always have eyewash stations checked and unblocked. Drills sound tedious, but the day an accident happens, everyone’s glad they did one last month.
Looking Ahead: Smarter Industry Practices
Technology keeps shifting toward safer alternatives, but industrial chemistry isn’t changing overnight. Companies should press suppliers for detailed toxicity data and safer ingredient mixes. More transparency means better risk assessment. Real workplace safety doesn’t end with compliance paperwork — it lives in honest conversations between managers and teams on the floor, in practical run-throughs, and in the daily work of respecting the materials we handle.
It’s Not Just Another Chemical on the Shelf
Octadecylbis(2-Hydroxyethyl)Methylammonium Chloride—a tongue-twister and a staple in several industrial and research labs—often finds itself tucked away quietly in storerooms. Most folks don’t spend much time worrying about where it sits, but the truth is, its long-term reliability depends a lot on how people store it. Neglect the conditions, and performance won’t keep up; in some cases, proper storage even sidelines serious risks.
Stay Cool, Stay Dry
Temperature swings play tricks on all sorts of chemicals, and this one is no different. Leaving it out under fluctuating summer or winter heat can cause caking, clumping, or even breakdown. The best bet? Store it somewhere at room temperature—around 20-25°C does the trick. Labs that keep their climate steady rarely run into trouble. Humidity, though, causes even more headaches. This compound, like most quaternary ammonium salts, likes to suck moisture from the air. If left open in a muggy storeroom, it turns sticky and starts to cake up. Keep the container sealed tightly and store it in a dry place.
The Container Counts
Experience in the lab quickly proves just any old jar won’t cut it. Once I saw a batch stored in a flimsy, re-used plastic bottle; after a month, bits flaked off and the top warped. That’s not just unsightly, it’s risky. Always go with a high-quality, airtight plastic or coated glass container. Inspect it before every use, and swap it out if it starts to look brittle or stained by previous chemicals.
Clean, Isolated Storage Makes All the Difference
Chemicals don’t play well together. Storing Octadecylbis(2-Hydroxyethyl)Methylammonium Chloride next to strong acids, alkalis, or oxidizers raises the risk of unwanted reactions. Segregating chemical storage might take up a little more shelf space, but it also means accidental cross-contamination can’t ruin your supply. Label the container clearly. If everyone on the team recognizes what’s inside, misuse or mistaken mixing happens a lot less.
Safety Isn’t Just a Box to Tick
I remember a near miss with a leaky bag stored beside an ammonium chloride stock. Nobody noticed the slow drip for days. Once those vapors mixed, the cleanup took hours—and set the schedule back a week. To prevent that, set the chemical on a spill tray and check the area often. Use gloves when handling, even if the material looks harmless. Eyes and skin feel the sting if it splashes.
Waste Management: Plan Ahead
Unused or spoiled Octadecylbis(2-Hydroxyethyl)Methylammonium Chloride doesn’t belong in the drain. It’s classed as hazardous waste in many jurisdictions, so collect leftovers in a clearly marked, compatible waste container. Connect with a licensed chemical waste removal service—don’t try to improvise disposal methods.
Keeping Everyone Accountable
Lab teams run smoother when everyone understands the importance of labeling, discarding expired material, and checking storage spots on a regular schedule. A quick checklist posted by the cabinet cuts forgetfulness. Taking ten extra minutes today can save hours of cleanup, wasted money, and potential risks down the road.
The Heart of the Molecule
Most people only come across names like Octadecylbis(2-Hydroxyethyl)Methylammonium Chloride by accident—maybe while reading an industrial cleaner label, or hearing about textile treatments that cut down on static. This compound might look like a chemistry test gone wild, but it has a clear function and a meaningful structure. Its chemical formula is C23H50ClNO2. Each character in that formula points to a real task in the molecule’s daily work: long hydrocarbon tail for surface activity, nitrogen for charge, a couple of hydroxyethyl groups for interactions.
What That Formula Signifies
Those twenty-three carbons, fifty hydrogens, one nitrogen, two oxygens, and a chloride ion tell a story. The long octadecyl chain (eighteen carbons in a row) is bulky, unyielding, and resists dissolving in water. That trait lets the molecule insert itself into oily messes or stick its tail into surfaces needing treatment. Friends in manufacturing call this a surfactant—lifting grease, helping fibers take on dyes, keeping rust from forming, even chasing away static.
Problems People Overlook
The trouble shows up when such compounds drift outside their intended use. This molecule breaks down slowly in the wild. Wastewater treatment plants run into challenges, since the highly charged nitrogen head sticks to sludge, not easily giving up its grip. Persistent residues build up. I’ve seen cases where quaternary ammonium compounds like this limit safe reuse of greywater in smaller communities. It can’t just be flushed away—in the end, all that extra nitrogen and non-breaking chains travel with the flow.
If you check the safety data sheets, toxicity toward aquatic life pops up now and then. Fish and small invertebrates don’t shake off this stuff. And yet, many companies lean on these chemicals for antimicrobial properties in disinfectants, especially after big outbreaks or during virus-driven cleanups.
Better Choices, Smarter Use
Folks who work with these materials day-to-day—janitors, textile workers, even farmers—depend on their reliable results. That reliability shouldn’t mean locking ourselves in to “the way we’ve always done it.” There’s talk now about greener surfactants, enzymes, or even biodegradable ammonium alternatives. I’ve watched a few industrial teams cut their environmental discharge in half simply by rethinking concentrations and limiting applications to truly high-risk areas.
Regulators and product designers have options. Clear labeling about breakdown times, water toxicity, and proper disposal instructions give people a real chance to use products wisely. On the shop floor, training helps. Some companies already check drainage systems to catch run-off loaded with quats. Others look at carbon footprints across supply lines, not just factory floors.
Science in Everyday Life
For anyone not deep in chemistry, the formula C23H50ClNO2 stands as a signpost—a reminder that even long words on plain labels point to choices that stretch from production to streams and rivers. Chemical literacy matters, not as trivia, but as a skill for safer homes and cleaner industries. More students learning what these formulas mean might ask tougher questions and push for changes with their own careers. That’s where real progress happens.
Looking Beyond the Label
At first glance, Octadecylbis(2-hydroxyethyl)methylammonium chloride might look like just another mouthful of a chemical name. But this compound shows up in real-life practical settings. Industry uses it for its antistatic and disinfecting properties, and you’ll spot its cousins in textile factories and treatment plants. The problem comes once it’s done its job—how should we deal with the leftovers?
The Risks Lurking in Fast Disposal
Letting unused chemicals go down the drain feels easy, but this thinking brings trouble. Wastewater systems are meant to filter out some nasties, but they rarely catch them all. Octadecylbis(2-hydroxyethyl)methylammonium chloride belongs to the group of quaternary ammonium compounds—these chemicals can damage aquatic environments even at low concentrations. Fish, algae, and invertebrates may not bounce back after repeated, invisible exposure. I’ve heard stories from those working near treatment plants, watching fish populations dwindle, and the scientists confirm the connection.
Mishandling also puts people at risk. Some quats cause skin, eye, or track irritation. The label might only warn about major mishaps, but even small spills in regular trash or down the drains can cause issues for sanitation workers, plumbers, or anyone downstream.
Smarter Practices for Industrial and Laboratory Waste
In my experience, proper disposal hinges on clear, enforced rules and people willing to follow them. Most industrial setups and university labs can’t dump waste directly. Rules require collecting chemical leftovers in clearly labeled, leak-proof containers. Many sites set aside lockable cabinets or rooms for chemical storage, away from heat and sunlight. Documentation matters, too—writing down what goes into each container reduces mistakes.
Specialized waste companies pick up these containers. Workers trained in hazardous materials take the containers to chemists who handle separation, treatment, or incineration. High-temperature incineration remains one well-established disposal method for this compound, breaking it down into less hazardous substances. I’ve watched teams suit up, check container seals, and double-check manifests—nobody wings it. Regulations such as the Resource Conservation and Recovery Act (RCRA) in the U.S. back up these practices with real consequences for cutting corners.
Options for Smaller-Scale or Household Scenarios
Rarely, a classroom or a DIY enthusiast encounters this chemical. Most regular trash services or wastewater systems won’t safely deal with it. Responsible disposal involves calling a local hazardous waste center or the relevant municipal authority. These centers often collect unused or expired chemicals on “household hazardous waste” days. I once took an old jug to a city collection drive—signs and workers guided me, and the peace of mind was worth the trip.
Room for Progress: Manufacturer Take-Back and Green Chemistry
Manufacturers could do better. Some lead the way by setting up take-back programs, especially if they sell drums of the stuff to smaller clients. Others supply clear, straightforward disposal instructions with their products. On the research side, green chemistry advocates keep pushing for safer alternatives—chemicals that give the same results without leaving environmental scars. Support for tighter regulations and ongoing education helps everyone make smarter decisions from the start.
Real Responsibility Means Facing the Chemistry
Disposing of Octadecylbis(2-hydroxyethyl)methylammonium chloride safely isn’t just technical red tape. It calls for practical steps, shared accountability, and a respect for the world downstream. Every bottle or container handled the right way keeps problems from spreading beyond the lab, factory, or workshop, and that’s a job worth doing properly.