Tert-Butyl 10-Bromodecanoate: A Deep Dive into Its Science and Impact
Historical Development
Chemists started experimenting with brominated fatty acid derivatives in the late 20th century, looking for new synthesis routes and reaction platforms that natural fats just couldn't offer. Discovery of Tert-Butyl 10-Bromodecanoate followed a long arc that began with the manipulation of simple esters and halogenated compounds in organic laboratories throughout Europe and North America. Early literature traces its roots to broader explorations of esterification and halogenation in the 1970s and 1980s. Researchers developed this compound to serve both as a synthetic building block and as a specialty reagent, recognizing the potential of the tert-butyl ester to safeguard carboxylic acids during tricky chemical reactions. My time spent leafing through yellowed research journals at university drove home how much innovation builds on small, incremental lab breakthroughs—one by one, researchers added tools like this to the organic chemist's toolbox.
Product Overview
Tert-Butyl 10-Bromodecanoate stands as an ester featuring a ten-carbon chain capped with a bromine at the omega end and a tert-butyl ester at the alpha end. Unlike simple methyl or ethyl esters, the tert-butyl group brings steric bulk, blocking unwanted side reactions and improving stability during multi-step syntheses. Chemists working with fatty acid chains appreciate how this molecule lets them add a reactive handle—bromine—exactly where they want, without risking the acid group’s reactivity. This compound often comes up on catalogs dedicated to advanced organic research, and shows up again and again in technical reports focused on long-chain modifications.
Physical & Chemical Properties
Looking at Tert-Butyl 10-Bromodecanoate in the lab, the liquid displays a faint yellow tint, flowing readily at standard temperatures. It gives off a mild, oily odor that’s common to brominated aliphatic esters. Measuring density, chemists find it falls in a predictable range: heavier than non-brominated esters but still lighter than many aromatic compounds. Its boiling point sits high due to the long carbon chain, topping 320°C before significant decomposition. The molecule resists hydrolysis under mild pH but will eventually break down to decanoic acid and tert-butanol if pushed hard—chemists have to watch out for this during storage, choosing dry environments and amber vials. Bromine’s presence makes the compound notably more reactive, especially with nucleophiles, while its large, greasy tail keeps it soluble in most organic solvents.
Technical Specifications & Labeling
A typical label for Tert-Butyl 10-Bromodecanoate names it by its IUPAC title: tert-butyl 10-bromodecanoate, often listing a CAS number to let researchers confirm its identity and avoid confusion with shorter or longer homologues. Bottle labels call out purity, usually sitting above 97%, and mention storage recommendations—keep in cool, dry, protected conditions. MSDS documents supply H-phrases warning about irritation or environmental hazards, while technical spec sheets list GC or NMR data for quick batch verification. Chemical suppliers include shelf life and container type, a must in labs where dozens of similar-appearing bottles line the shelves. Transparency about product source and manufacturing process has grown—something I’d never seen as a student, but common now due to regulatory and quality control changes since the 2010s.
Preparation Method
Synthesizing Tert-Butyl 10-Bromodecanoate means combining traditional organic tricks. Chemists start with 10-bromodecanoic acid, a product of controlled bromination on decanoic acid. Careful use of bromine and specific radical initiators avoids over-bromination or carbon backbone degradation. With the acid in hand, engineers react it with tert-butanol using an acid catalyst—often sulfuric or p-toluenesulfonic acid—to drive esterification. Purification often includes distillation under reduced pressure and column chromatography to remove leftover acid, unreacted alcohol, and side products. Procedures call for skill; amateur mistakes cause overheat, water contamination, or incomplete reactions, risking frustrating, low-yield batches.
Chemical Reactions & Modifications
Tert-Butyl 10-Bromodecanoate opens doors to functional group transformations. The terminal bromine behaves as a robust leaving group, well-suited for nucleophilic substitutions—chemists routinely replace it with azides, thiols, amines, or alkoxides, turning out products for further development or pharmaceutical use. Deprotection of the tert-butyl ester, usually achieved with acid or strong Lewis acids like boron trifluoride, releases free 10-bromodecanoic acid when needed. These features make the compound a handy intermediate for the invention of surfactants, specialty polymers, and custom-tailored molecules. Peers I knew during my own PhD worked with similar esters to template advanced surfactants and drug delivery vehicles, leveraging both the bromine's reactivity and the stability of the tert-butyl cage.
Synonyms & Product Names
This compound appears across journals, datasheets, and catalogs under several identifiers: tert-butyl 10-bromodecanoate, 10-bromodecanoic acid tert-butyl ester, or sometimes as t-Bu 10-Br-decanoate. Chemists use shorthand like C10Br–OtBu on whiteboards or in working notebooks, but suppliers stick to more formalized names to keep legal and regulatory documents in line. Misspelled or mistyped synonyms in order forms occasionally create headaches—accuracy matters when working with such tailored reagents, and cross-checking CAS numbers remains critical for avoiding misorders.
Safety & Operational Standards
Anyone using Tert-Butyl 10-Bromodecanoate follows well-worn safety standards. Direct contact causes skin and eye irritation, so gloves, goggles, and good ventilation rank as basic requirements. The ester’s vapors—though not overpowering—still demand careful handling away from open flames due to both its flammability and its brominated byproducts, which can turn nasty if mistreated. Disposal procedures line up with environmental rules covering halogenated organic waste, reflecting a steady tightening of chemical handling regulations over the last two decades. Labs today operate under robust SOPs—every spill gets reported and logged, every container traced and tracked. Hearing stories from older chemists about their freewheeling days at the bench makes me realize how much the field has changed for the better, prioritizing both human and environmental health.
Application Area
Industries reach for this ester mainly as an intermediate for synthesizing more complex, functionalized materials. The pharmaceutical world values it for building long-chain analogs with precisely controlled modifications; the bromine’s position unlocks routes to tailored lipids and modified surfactants. Chemical manufacturing looks at this molecule for developing specialty polymers or for creating advanced lubricants that can handle high-stress, low-foaming environments. Research institutions continue digging into Tert-Butyl 10-Bromodecanoate for making specialty probes or tracking how fatty acid derivatives change biological membranes—projects that blend chemistry, material science, and even biophysics, as researchers hunt for answers about cell structure or smart materials.
Research & Development
R&D teams focus on expanding what can be done with this kind of molecule. Exploring optimized syntheses, researchers lower waste, switch to greener solvents, and hunt ways to make reactions more energy efficient. There’s a constant push for new derivatizations—methods for introducing fluorine, extra aromatic rings, or bioactive tags—driven by pharmaceutical development for targeting disease pathways. The compound’s utility for click chemistry platforms, enabling modular addition of tags or probes, keeps it on the radar for those working on diagnostics and targeted therapies. I recall colleagues using similar substrates to modify nanoparticles, opening up routes to drug delivery methods not imaginable just a decade ago.
Toxicity Research
Toxicological studies on Tert-Butyl 10-Bromodecanoate remain mostly preclinical, but the structure suggests some risk from both the brominated chain and its breakdown products. Animal studies point toward irritation and possible liver effects at high exposures; the tert-butyl group can release isobutylene under harsh metabolic conditions, which researchers track when studying chronic toxicity. Environmental toxicology branches out further, evaluating risks to aquatic organisms and the breakdown pathways in soil and water. Regulatory bodies now require detailed ecological impact reports for any compound with halogen substitutions. I’ve seen whole conferences devoted to new testing methods that provide rapid, meaningful toxicity data for compounds just like this one, highlighting the industry’s evolution from basic physical tests to integrated biological models.
Future Prospects
The outlook for Tert-Butyl 10-Bromodecanoate connections with larger pushes in green chemistry and tailored molecular design. Advancements in catalysis hold promise for making reactions more sustainable, possibly using less aggressive reagents and recyclable catalysts. As polymer and surfactant research veers toward custom performance additives, demand for well-defined omega-functionalized intermediates will keep rising. Artificial intelligence opens new possibilities for predicting reaction outcomes and guiding experimental design; this trend will touch every aspect of specialty chemical development, from batch synthesis to toxicity prediction. More transparent reporting and traceability also look likely to become the new baseline—an adjustment that reflects public and regulatory interest in cleaner, safer chemistry, and one I support wholeheartedly from both an environmental and operational perspective.
Looking at the Molecule Up Close
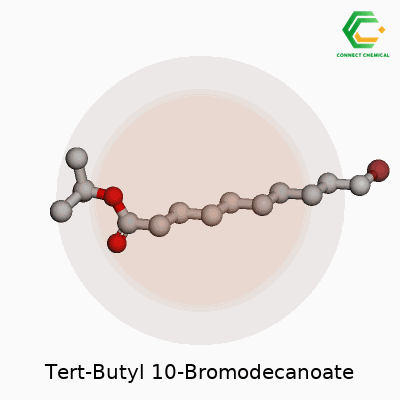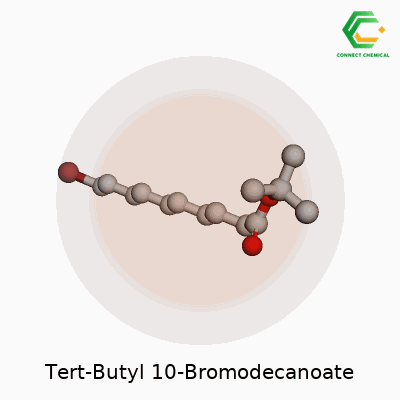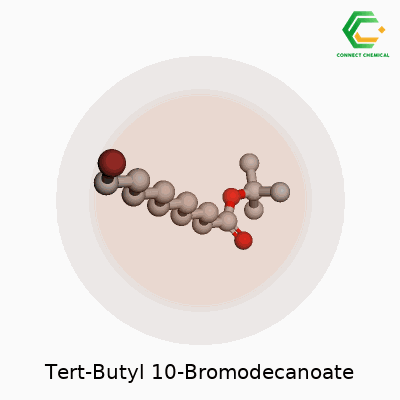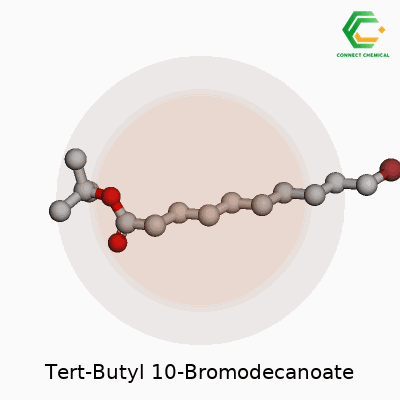
Organic molecules aren’t just random bunches of atoms thrown together. Every bond, every branch, every addition affects what a chemical can do in the real world. Tert-butyl 10-bromodecanoate is a fine example—showing off how a handful of tweaks to a long carbon chain give rise to a whole new set of possibilities. Start with the backbone: a ten-carbon chain. One end gets a tert-butyl ester group. The other holds on to a single bromine atom. This setup causes unique traits both in the lab and outside.
Chemical Structure Details
Picture this molecule: the decanoate refers to a chain of ten carbons with a carboxylate group. Attach a tert-butyl group to the oxygen on that carboxylate, creating the tert-butyl ester. On the opposite end, at the tenth carbon, swap a hydrogen for a bromine atom. The formula looks like this: C14H27BrO2.
The tert-butyl ester group, known for bulk and stability, shields the carboxylic acid from easy reactions. This is no accident. Labs use tert-butyl esters to guard acids during long synthetic processes because they hang together through most reaction conditions but come off cleanly with acid. Sitting there on one end, it keeps the molecule in check. At the other end, the bromine offers a handle. Chemists tend to use this spot in substitutions or couplings, building out new compounds by kicking out the bromine and replacing it with something else.
Why It Matters to Have an Exact Structure
Whether designing a pharmaceutical, a specialty material, or something for a food additive, no one can fake their way through without grasping the underlying chemistry. Add a bulky tert-butyl group, and a molecule sidesteps water for a while. Drop in a bromine, and the molecule becomes much more reactive at that spot. These changes lead to better control over when and how a molecule transforms, making it possible to get the right structure for the experiment or product in mind.
Synthetic work depends on predictable reactions. Without a clear structure, things get out of hand quickly. With tert-butyl 10-bromodecanoate, researchers can set up a reaction, trusting that the ester will hold out and the bromine will swap out just as planned. This reliability helps save time and money, avoiding ruined experiments and wasted supplies.
Ways to Tackle Common Challenges
Throughout chemical production—to make something like this reliably—teams often run into trouble with purity, yields, and environmental impact. Leftover brominated side-products, incorrect placement of the protective ester, and harsh deprotection steps can crop up fast.
Improvement starts with cleaner synthetic routes. Solid research points toward milder reagents and greener solvents. Using phase-transfer catalysts has cut down reaction times and lowered energy use in several labs. Quality control should not play second fiddle. Analytical techniques, including NMR and mass spectrometry, catch impurities before they become a problem.
Deprotection, the step where the tert-butyl group eventually comes off, grants precision. Strong acids like trifluoroacetic acid remove the group without scrambling the rest of the molecule. Consistent results require tight grip on temperature and acid concentration.
For anyone developing new molecules, mastering the subtleties of a structure like tert-butyl 10-bromodecanoate lays a solid foundation for innovation. Each group on the chain serves a purpose, not just in academic exercises, but in the marketplace products that shape daily life.
Building Blocks in Chemical Synthesis
Some chemicals play bigger behind-the-scenes roles than most people think. Tert-Butyl 10-Bromodecanoate falls into that category. Folks in organic chemistry often turn to this compound to build up more complex molecules. Its straight carbon chain topped with a bromo group offers chemists a strong base for making specialty chemicals. The tert-butyl part at the end helps protect the molecule during reactions, so the key features of the chain stay intact through complicated chemical steps.
From my experience in lab-based research, this molecule shows up a lot when the goal is to introduce an alkyl bromide group at a specific spot on a carbon chain. It sets the stage for creating something more complex. Researchers count on its stability and easy-to-handle properties, especially in academic and industrial labs working on custom syntheses.
Pharmaceutical Intermediates
Drug discovery efforts benefit from specialty reagents, and Tert-Butyl 10-Bromodecanoate pulls its weight here. Medicinal chemists use it as an intermediate to construct bioactive molecules, often connecting it with other fragments through well-known reactions. For example, it helps create linkers or spacers in molecules that target disease, such as modified fatty acid derivatives or prodrugs.
A team I once collaborated with relied on this compound to make a series of ester-linked pharmaceuticals. The tert-butyl ester group kept parts of the molecule shielded, so the main chain could withstand harsh conditions. They could later remove the tert-butyl group under mild settings, leaving a clean product ready for further modification. This efficiency can speed up the process of new drug design.
Material Science and Surface Modification
Materials scientists try to fine-tune how surfaces interact with their environment. Tert-Butyl 10-Bromodecanoate plays a role in surface treatment. Its long carbon tail lets it anchor to surfaces, while the bromo group works as a chemical handle for attaching new groups. This design allows for the creation of advanced coatings, including anti-fouling layers or surfaces that interact with living cells in specific ways.
During a stint at a materials lab, I saw colleagues use this compound to create densely packed monolayers, which they then modified to add different chemical features. This method improved both performance and life span of coatings in real application tests.
Specialty Polymers
Manufacturers of specialty polymers look for monomers with reactive handles. Tert-Butyl 10-Bromodecanoate brings both a protected ester group and a bromide, which can initiate further reactions like substitution or elimination. This combo makes it useful when designing copolymers or block polymers that demand chemical precision.
Production teams have used it to introduce specific points for cross-linking, which boosts strength or adds flexibility. These tailored materials can show up in medical devices, electronics, or adhesives. Advanced manufacturing relies on reliable and well-understood reagents, and Tert-Butyl 10-Bromodecanoate proves its worth here.
Looking Ahead
There’s always room to improve how chemists make and use specialty intermediates like this one. Manufacturers might shift toward greener routes or switch to renewable feedstocks to cut their environmental impacts. Chemists can explore alternative protecting groups or reagents, especially to avoid hazardous byproducts or simplify purification. Everyone who works with Tert-Butyl 10-Bromodecanoate stands to gain from smarter, safer, and cleaner synthesis practices.
The Role of Purity in Chemical Sourcing
For anyone working in synthetic chemistry or materials science, purity of reagents shapes outcomes. In my early days in a university lab, nothing taught me the value of purity more than watching two parallel syntheses veer in different directions because of subpar starting compounds. Tert-Butyl 10-Bromodecanoate stands as a solid example—get the purity wrong, and your downstream chemistry suffers.
Typical Specification and What It Means
Reputable suppliers usually offer Tert-Butyl 10-Bromodecanoate at a purity specification of not less than 97%, often topping out at 98% or even purer batches for more sensitive applications. This is usually confirmed by GC or HPLC analysis, backed by a thorough Certificate of Analysis. Experienced chemists will not trust a drum or bottle unless it tells them what the remaining 2-3% actually contains.
Impurities in this product typically look like residual solvents, minor byproducts from bromination such as over-brominated species, trace acids, and any leftover starting materials. Each impurity carries risk: unexpected reactions, lower yields, and unpredictable toxicity. Even so-called “minor” differences can mean the difference between a high-yielding, clean process and an expensive failure.
Safety Goes Hand-in-Hand with Purity
Laboratory safety officers get antsy for good reason. Unknown impurity profiles raise the danger in chemical reactions—not just ruined experiments but potential exposure to corrosive, carcinogenic, or allergenic materials. Any experienced chemist will tell you that transparent, rigorous purity testing isn’t just “nice to have.” It’s necessary for safe and predictable handling.
A few years ago, my team attempted a scale-up using what turned out to be a low-purity batch. A cloud of noxious fumes triggered alarms, halting an entire building. Looking back, all of it pointed to poor impurity characterization.
Choosing Wisely: Navigating Purity Challenges
The price tag of high-purity Tert-Butyl 10-Bromodecanoate can tempt budget-minded project managers into cutting corners. The reality is, small savings here often snowball into oversized troubleshooting costs down the road. Reliable suppliers should provide not just a percentage figure, but a breakdown of known impurities, solvent residues, and water content.
Raw data should match what’s in the spec sheet. If a supplier refuses to share chromatograms or detailed impurity profiling, that’s more than a red flag—it’s a warning siren. As someone who’s screened dozens of vendors for custom esters over the years, I would rather pay a premium than lose weeks cleaning up after hidden contaminants.
Fact-Based Decisions Support Good Science
Any scientific publication or process validation using Tert-Butyl 10-Bromodecanoate must reference the actual purity along with supporting analytical data. Peer reviewers, collaborators, and regulators all expect this level of disclosure. Nobody wants to see their work questioned because of doubts about their starting materials.
Project leaders and technical buyers gain confidence by pushing for complete certificates and third-party verification. Real trust comes from a history of data-backed sourcing, not from slick brochures or persuasive sales reps. Building relationships with trustworthy suppliers helps avoid setbacks and enhances reproducibility—a crucial pillar in both commercial R&D and academic research.
Practical Steps Forward
Anyone working with Tert-Butyl 10-Bromodecanoate should make purity documentation standard practice. Keep communication open with suppliers about analytical methods and impurity risks. Run your own checks on received materials and treat every unknown as a potential disruptor. These habits drive better results—not just in yield, but in safety, reliability, and scientific credibility.
Treating Chemicals With Respect
Anyone who keeps chemicals knows a small mistake can turn into a big problem. Tert-Butyl 10-Bromodecanoate gets used by lab workers, chemists, and manufacturers for synthesis or research. This stuff does not belong on a regular shelf. I learned early on during my lab jobs that proper storage keeps both people and projects safe. Too many times, I saw chemicals lose potency or, worse, cause surprise reactions when handled the wrong way.
Why Storage Matters for This Compound
Storing Tert-Butyl 10-Bromodecanoate inside a tightly sealed glass or compatible plastic bottle is step one. Bromine-containing substances like this one react with moisture, so a well-sealed container blocks out humidity. If even a little water gets in, you risk decomposition, and then the bottle's contents just become a liability. Using new containers always gave me more peace of mind than recycling old bottles that might hold invisible residues.
Temperature and Light: The Hidden Enemies
Heat destroys sensitive organic intermediates fast. This compound needs a cool spot—something like 2–8°C, the kind of range you see in standard lab refrigerators. Leaving it near sunlight or a hot radiator never ends well. UV rays or high heat spark unwanted chemical changes. I made it a practice to label bottles with “Light Sensitive” stickers, which kept forgetful hands from leaving the container uncovered near windows. Even fluorescent lab lighting sometimes triggers slow degradation, so darkness truly helps.
Isolation Prevents Accidental Cross-Reactions
Many labs cluster all their reagents together, hoping for convenience. A few months into my first real research job, I watched someone stack incompatible reagents on one shelf. One solvent cap leaked, and next thing you know, smoke poured from the shelf. I never forgot the panic. Store Tert-Butyl 10-Bromodecanoate away from acids, bases, oxidizers, or anything reactive. Dedicated shelves or bins in a chemical storage fridge keep surprises to a minimum.
Safety Isn’t Optional
No one wants to discover a chemical with the label worn off or see the safety data sheet gone missing. I started printing waterproof labels and taping laminated instructions nearby after seeing confusion cost valuable time. Proper documentation—right next to the storage area—cuts down on error. Gloves, goggles, and a well-ventilated work area remain non-negotiable. Brominated esters sometimes sneak out a faint odor or leak vapor, and exposure brings both health risks and liability headaches for the lab manager.
Staying Prepared for the Unexpected
Spills do happen, and every lab that stores chemicals should have a spill kit nearby. Simple steps like using secondary containment bins and weekly bottle checks prevented more than one mess in places I worked. It takes minutes, but those minutes can save hundreds of dollars and prevent avoidable injuries.
Building Lasting Good Habits
Quality work in research or manufacturing only finds solid ground on good habits. The right storage—dry, cool, dark, isolated, and documented—keeps both people and products safe. After handling chemicals for years, I know cutting corners doesn’t pay off. Taking a little extra care every time gives peace of mind and shows respect for the craft. Any facility handling Tert-Butyl 10-Bromodecanoate can do better by following clear, practical, well-informed routines.
Navigating the Search for Bulk Specialty Chemicals
Anyone who has spent time sourcing specialty chemicals for research, pharmaceuticals, or advanced materials will know the pain of tracking down niche compounds like tert-butyl 10-bromodecanoate. Years in labs and close work with procurement teams shaped my sense of what it takes to secure obscure intermediates at scale. Bulk demand rarely comes from curiosity—it means a business or research project could stall if the right supplier isn’t ready.
Understanding Supply Trends
Supply for tert-butyl 10-bromodecanoate ties straight to its role in organic synthesis and pharma applications. Companies synthesizing custom molecules, particularly those targeting modified fatty acids or building block esters, lean heavily on intermediates like this. Data floating around industry reports and specialty supplier websites shows a concentration of production in select regions. Most suppliers—primarily in China and India—serve inquiries for limited research quantities; their catalogs show grams and small multi-gram packs, not drums or 25 kg sacks.
Custom synthesis has taken over the territory left behind by traditional in-house chemical manufacturing. Several contract manufacturers offer this compound, but their lead times and price quotes can raise eyebrows. A supplier might confirm the ability to deliver 10, 20, or even 100 kilograms, though they’ll warn about synthesis delays, custom packaging, and potential export restrictions dealing with brominated compounds.
According to ChemSpider and Sigma-Aldrich, tert-butyl 10-bromodecanoate isn’t a regular shelf item in bulk. A few small manufacturers in Eastern Europe and South Asia published lots ready to ship upwards of 5 kg. Conversations with procurement contacts showed the reality: most “in stock” claims vanish once you ask for anything over standard lab use. The provider often checks their partner network, then comes back with a fresh lead time, typically 4 to 8 weeks. Price escalates quickly, reflecting both the custom labor and transport involved.
Why Bulk Sourcing Stumbles
Global regulations on hazardous substances complicate logistics. Brominated molecules often get flagged in customs, leading to unexpected delays or additional paperwork. Many labs in the US and EU work with local distributors to avoid the headaches of direct import. Middlemen add cost but cut risk—and compliance headaches. Large-volume buyers sometimes balance cost and security by splitting orders among several distributors, hoping that at least one delivers on time.
Quality is another issue. Bulk batches can introduce surprise impurities if production batches aren’t controlled tightly. In my experience working with pharmaceutical firms, authenticated certificates of analysis are non-negotiable—a single impurity can blow up months of R&D or require expensive cleanup. Bulk orders often require in-person audits of overseas plants or third-party verification, making the timeline extend even more.
Solutions for the Industry
Direct relationships with proven custom synthesis shops nudge this picture in a better direction. Regular communication, in-person visits, and repeat orders allow buyers to pick up early warnings about supply crunches or regulatory changes. Some top contract manufacturers have started holding modest buffer stocks of high-demand intermediates, including brominated esters, creating more predictable delivery for their priority buyers.
Digitization—the ability to get real-time inventory checks, updates on synthesis progress, and track shipping from factory to door—has made sourcing less of a black box. Digital marketplaces have started connecting buyers directly to small-batch manufacturers in Asia and Europe, slashing sourcing times for highly specialized chemicals. Sourcing tert-butyl 10-bromodecanoate in bulk still calls for persistence and savvy negotiation, but a more transparent market offers hope for researchers and manufacturers facing similar roadblocks.