Tert-Butyl 4-Bromobutyrate: Unpacking a Modern Building Block
Uncovering the Roots: The Path to Tert-Butyl 4-Bromobutyrate
Back in the early days of organic synthesis, chemists saw the challenge of building bigger molecules from simpler, readily available pieces. The rise of organobromides during the late 20th century really changed the lab landscape, opening doors for targeted modifications and clever routes to complex compounds. Tert-Butyl 4-Bromobutyrate sits in that tradition, arising from a blend of practical curiosity and the need for safer manipulations. Researchers wanted a protected ester that reacts cleanly, survives tricky conditions, and still offers a good handle for nucleophilic substitution. This particular compound owes a lot to the story of protecting groups and the constant push for purer, more manageable intermediates in both pharma and research labs.
Breaking Down the Product
You find a clear, colorless to pale yellow liquid when you open a fresh bottle of tert-butyl 4-bromobutyrate. Its slight fruity odor often reminds me of countless late nights in the synthesis lab, trying to coax stubborn reactions forward. Each batch, usually tagged with over 95% purity, comes with a guarantee of major chemical compatibility—easy solubility in common organic solvents like dichloromethane, diethyl ether, and ethyl acetate makes it fit neatly into almost any workflow. For most users, the real selling point comes from its versatility: chemists use tert-butyl 4-bromobutyrate as both a masked acid and a source of a reactive alkyl bromide, giving it double utility in multi-step synthesis.
Physical and Chemical Highlights
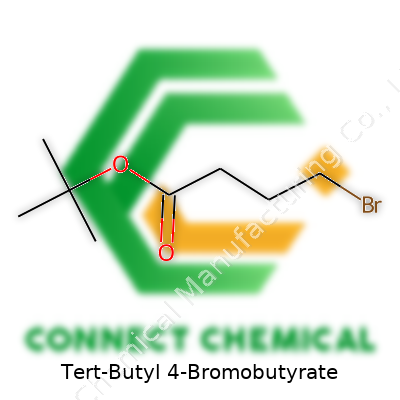
This molecule—C8H15BrO2—combines the bulky protection of a tert-butyl ester with the electronegative snap of a bromine atom. With a molar mass of about 223 g/mol, it doesn’t win any heavyweight awards, but the practical boiling point hovers around 78–80°C at reduced pressure, and it can withstand storage under nitrogen for months without decomposing. The bromine atom on the tail of the butyrate chain reacts quickly and selectively, serving as a nearly perfect electrophilic site. For shelf life, as long as you cap the bottle tight and keep it in a cool, dry corner of the fridge, it resists hydrolysis and oxidation quite well.
Labeling and Technical Details
Bottles always arrive covered with hazard diamonds, reflecting the reagent’s mild toxicity but also nodding to the importance of careful handling in any organic lab. Technical specs focus on purity, water content (should be kept under 1%), and color (typically APHA color less than 30). Modern quality standards, especially those meeting pharma or ISO requirements, demand every drum or vial carry clear batch numbers, certificate of analysis, and, if shipped internationally, transport info for brominated organics.
Synthesis in Practice
In the lab, two paths generally lead to tert-butyl 4-bromobutyrate. One common route starts with succinic anhydride, transformed with tert-butanol under acid catalysis into tert-butyl 4-hydroxybutyrate. Next, a bromination step, often using phosphorus tribromide or hydrobromic acid, swaps out the hydroxyl for a bromine. Choosing reagents and conditions always involves a careful balance—ensuring high yield without damaging the fragile tert-butyl ester, and keeping the final product free of water and acidic byproducts. Processes developed in the 1990s improved yield and minimized waste, turning what used to be a tricky and expensive intermediate into a regular item found on most chemical supplier shelves.
From Transformation to Tool: Chemical Reactions and Modifications
Tert-butyl 4-bromobutyrate plays a starring role in substitution and alkylation reactions. The bromine atom leaves readily, letting all sorts of nucleophiles—from azides to amines—swap in and create new molecules. The tert-butyl ester withstands strong bases and mild acids, which means chemists can fiddle with different parts of the molecule before finally “unmasking” the acid group via acid-promoted hydrolysis. This two-step utility streamlines peptide synthesis and the design of prodrugs, making the molecule far more than a simple reagent—it acts as a strategic enabler in custom synthesis.
Other Names and Aliases Across the World
Known among chemists as tert-butyl 4-bromobutyrate, you’ll also see its synonyms pop up in catalogs and literature: 4-bromobutyric acid tert-butyl ester, 4-bromobutyric acid t-butyl ester, and even TB4BB for shorthand. CAS Number 90235-11-7 marks it in regulatory databases, but naming differences come mostly from linguistic habits or supplier branding. These alternative names can trip up even seasoned order clerks, making a proper chemical identifier essential for international sourcing.
Lab Practice: Safety and Handling
Every bottle deserves respect. Glove up before use, and keep work under a fume hood. While tert-butyl 4-bromobutyrate doesn’t emit clouds of choking vapor, liquid contact with skin or eyes stings and may sensitize over time. Spills call for activated charcoal or sand, scooping up promptly to avoid any lasting contamination. Waste streams need halogenated solvent bins, never a regular drain. Labs routinely train staff to check labels, avoid open flames, and stow containers below eye level. Safety Data Sheets underline the big points: not acutely toxic at lab scale, but repeated exposure or careless use can cause chronic irritation or environmental problems.
Where Tert-Butyl 4-Bromobutyrate Finds a Home
You spot this ester in the toolkits of peptide chemists and medicinal scientists designing new drug scaffolds. In my own work, the compound enabled the introduction of protected carboxylates onto bioactive molecules, letting us tinker with prodrugs that only activate under acidic conditions in the body. Chemical biology groups favor the bromide’s leaving group ability in tagging or linking biomolecules, while material scientists find use in polymer synthesis when they need a functional monomer that survives processing but can be unveiled down the road. Production of pharmaceuticals, especially beta-lactam antibiotics and central nervous system agents, sometimes picks up intermediate steps built around this tert-butyl-protected route.
Current Investigations and R&D Directions
Researchers keep poking at ways to boost green chemistry profiles—studies look into cleaner bromination methods and catalytic alternatives. Having environmental safety always in mind, new work tests less toxic bromine sources and recyclable reaction media. The major thrust in industry leans toward scaling reactions while squeezing out as much waste as possible. Pharmaceutical teams never stop looking for new prodrug moieties, and tert-butyl esters still rank among the more reliable. Techniques like continuous flow synthesis improve consistency and open up possibilities for automation—improving reaction times and purity beyond what I ever saw in my grad school days.
Toxicity Research: What’s Really at Stake?
Laboratory safety officers demand a close look at how the compound affects cells and organisms. So far, studies point to mild, manageable toxicity: the main threat comes from skin, eye, or respiratory exposure in concentrated form. Animal models show rapid metabolic breakdown, mostly safe at low doses typical of lab work, but repeated high exposure can stress kidneys and liver. Environmental research highlights persistence, since brominated compounds often linger if not disposed of properly. The drive continues for more detailed data, as regulators in the EU and US want clear toxicological profiles for all synthetic building blocks heading downstream into consumer products. Responsible labs now invest heavily in real-time monitoring and updated protocols every few years.
What’s Next for Tert-Butyl 4-Bromobutyrate?
Demand grows in step with the expanding universe of synthetic biology and medicinal chemistry. Advances in automated synthesis, greener reagents, and tighter regulatory frameworks put pressure on suppliers to rethink packaging, traceability, and safety documentation. I suspect breakthroughs in catalytic chemistry and non-halogenated alternatives will set the tone for years ahead—but the reliability, versatility, and well-known reactivity of tert-butyl 4-bromobutyrate ensure it holds a key place in the creative toolkit of chemists for the foreseeable future.
A Look at the Structure
Tert-Butyl 4-bromobutyrate isn’t the kind of compound most people talk about at the dinner table but, in the world of chemistry and pharmaceuticals, it packs plenty of importance. Its chemical formula reads as C8H15BrO2. This short line tells a big story for anyone who works with organic synthesis. The backbone contains a four-carbon chain with a bromine atom hanging off the end (the “4-bromo” part), capped with a tert-butyl ester group. This structure gives the compound unique properties—both in reactivity and stability.
Why Structure Shapes Purpose
Formulas like C8H15BrO2 aren’t just about counting atoms. Each piece signals a practical trait. The bromine brings a handle for chemists to make new bonds. The tert-butyl part shields certain reactions, letting professionals control what happens and when. I’ve seen this compound used in the lab as a key intermediate—a stepping stone—on the way to making more complex molecules, including some that can treat real human diseases. The formula may look dry, but it connects straight to discoveries that land in hospital pharmacies and research papers.
Building Blocks in Drug Development
Every drug journey kicks off with simple starting materials. Tert-Butyl 4-bromobutyrate sits comfortably on the shelf for those building nervous system agents, antivirals, or experimental new treatments. Chemists count on its reliable reactivity. The tert-butyl group blocks parts of the molecule, steering the reaction away from those spots. This trick helps direct the chemistry exactly where it’s wanted, saving time, money, and precious sample material.
Why Quality and Purity Matter
There’s no shortcut around proper handling. Poor purification choices can introduce mystery byproducts. Even one wrong atom can wreak havoc, especially in pharmaceutical research. Facilities have started demanding analytics like NMR and HPLC profiles to double-check what sits in the flask. It’s not just about ticking a box—it protects investments and, eventually, patients. Tert-Butyl 4-bromobutyrate, thanks to its clean and straightforward formula, delivers predictability when manufactured with care.
Environmental Responsibility in Synthesis
The bromine in its formula grabs attention for another reason—it can raise environmental concerns. Organobromides are known to stick around in nature, sometimes causing harm. Responsible labs emphasize greener approaches—recovering solvents, properly neutralizing waste, and designing processes that use less hazardous reagents. Newer methods even aim to swap out bromine for less persistent alternatives, or to recycle spent material. Sustainable practice doesn’t just satisfy regulations; it helps safeguard the research community’s reputation and the wider world.
Solutions Through Smart Chemistry
Reliable sources, careful lab work, and responsible disposal all flow together when handling chemicals like Tert-Butyl 4-bromobutyrate. Communication among teams limits risk, and keeping up-to-date with improved synthesis strategies sharpens efficiency. Open-access data and published reaction methods let early-career chemists learn the ropes and sidestep costly mistakes. Nothing beats a simple, well-characterized formula as a foundation. And without the right starting point, even the brightest idea in medicinal chemistry might stall in the test tube. The story of this molecule’s formula echoes in every smart solution that follows after.
Chemistry in Action
Tert-Butyl 4-bromobutyrate doesn’t show up on TV ads or medicine bottles, but it puts in a lot of behind-the-scenes work in chemical labs. If you’ve spent any time in organic synthesis—or chatted with people who do—you know certain compounds help reactions go smoothly. One of its main jobs is as a building block in making more complicated molecules. This ester contains both a tert-butyl group and a bromine atom, which gives chemists a couple of useful handles to work with during multi-step syntheses. For people designing small molecules, especially in pharmaceutical research, having a reliable way to add a four-carbon spacer or to introduce a protected carboxylic acid group comes in handy. The tert-butyl group guards the acid part of the molecule, so it doesn’t take part in reactions until the chemist decides to release it—usually at a late stage. The bromine, on the other hand, is primed for nucleophilic substitution, letting researchers swap it for all sorts of new groups.
Making Pharmaceuticals and More
In the world of drug discovery, chemists use tert-butyl 4-bromobutyrate for making various intermediates. It helps them piece together fragments that go on to become antivirals, antihypertensives, and sometimes experimental cancer treatments. Its popularity grows out of its flexibility. Say you need to make a molecule with a flexible carbon chain and a carboxylic acid that needs to remain untouched until the last step, this compound makes that much easier. After the essential parts of the molecule lock into place, a simple treatment with acid or heat pops off the tert-butyl group, freeing up the acid for further functionalization.
Beyond pharmaceuticals, this chemical has a home in research and industrial chemistry. In my own graduate school days, using protected esters like tert-butyl 4-bromobutyrate made all the difference between a clean reaction and a stubborn mess. I’ve seen teams avoid weeks of troubleshooting by choosing this ester for its reliability and easy deprotection.
Protecting Groups and Synthetic Strategy
The principle behind “protecting groups” might look basic, but it can determine whether a synthesis runs or stalls. Complex chemical reactions run up against all sorts of detours: sometimes, an acid group reacts before you want it to or changes the path of your chemistry. By shielding that piece with a tert-butyl group, the rest of the molecule grows as designed. Only in the final step, when the finish line’s in sight, do chemists remove the protecting group. Tert-butyl 4-bromobutyrate stands out because it keeps the process efficient and reliable. This approach crops up in everything from new insecticides to polymers used in electronics.
Challenges and Solutions
Handling chemicals like tert-butyl 4-bromobutyrate comes with the usual lab concerns. Brominated compounds sometimes raise environmental eyebrows because of toxicity and disposal requirements. Researchers must follow strict protocols for storage, handling, and waste treatment. Companies look toward greener alternatives, but until those take over, responsible stewardship is key. Switching to less hazardous reagents or advancing new catalytic processes may help ease some of those worries. Expanding education around safe laboratory techniques keeps both professionals and students protected while letting discovery continue.
Daily Life Meets Chemical Reality
Not every household keeps bottles of Tert-Butyl 4-Bromobutyrate on the shelf. Still, in research labs and industrial chemistry, this compound pops up often. It plays its part in complex syntheses, especially when building blocks for pharmaceuticals and specialty chemicals come into play. From experience handling fine chemicals, most of the real risk comes after the shipment lands, as soon as the team starts opening containers and transferring to working volumes. Shortcuts in storage turn into headaches fast.
Recognizing Real Risks
Tert-Butyl 4-Bromobutyrate acts a lot like other organic bromides—volatile, flammable, and capable of causing problems for human health. Fumes can irritate the nose and throat. Leaks never just clean themselves up, and chemical burns or allergic reactions can happen after accidental contact. Facts back this up: several incidents in university labs ended with emergency cleanups or, worse, students visiting the campus medical office. A spill leads to exposure, and exposure brings bigger problems than just cleaning up a puddle.
Temperature, Light, and Moisture: Enemies of Stability
Open bottles, sunlight streaming across a workbench, and humidity create a recipe for degradation. This compound breaks down fast at warmer temperatures—the literature agrees, as product data sheets from established producers state shelf life drops sharply if left at room temperature for long stretches. Also, light can trigger unwanted reactions, forming side products and breaking the molecule into stuff no one can use. Even trace water from damp air starts to hydrolyze it, sometimes without visible warning signs until the bottle fizzles with pressure when opened.
What Actually Works: Practical Solutions for Storage
My own lab moved from the open shelf system to dedicated chemical cabinets backed away from windows. We saw a big drop in waste and exposure just by moving temperature-sensitive bottles into a refrigerator kept at 2-8°C. Seals on bottles must be double checked; a loose cap draws in moisture, and pretty soon, the bottom of the bottle shows flakes and cloudiness. Staff use amber glass to block stray light. If the bottle stays sealed tightly, with a small pack of desiccant nearby, we keep the contents clear and usable for much longer.
Separating Tert-Butyl 4-Bromobutyrate from incompatible compounds—strong bases, oxidizers—makes the whole area safer to work in. A splash of strong base in a chemical waste bottle will gas out a room in minutes. Chemical manufacturers recommend against storing with acids or bases, or even open air, for good reason. Labels on containers help, but the routine of checking shelf dates and verifying bottle appearance stops trouble early.
Training and Culture: More Important Than Fancy Equipment
No storage rule replaces common sense and shared vigilance. Training sessions at our site always highlight storage basics. New staff and students walk through how to spot bad storage before it causes harm. Every time we log a misshapen bottle or catch a cracked seal before it leaks, someone’s protected from potential chemical burns or ruined experiments. Chemical safety builds on simple, daily habits: respect the substance, respect your coworkers, and the rest falls into place.
A few minutes taken to store Tert-Butyl 4-Bromobutyrate the right way spares hours—sometimes days—of disasters and downtime. Fact-driven habits, not just checklists, raise the standard for everyone working with hazardous chemicals.
Tackling Chemical Safety, One Compound at a Time
Every lab worker eventually bumps into odd-named chemicals. Some sound sinister—tert-butyl 4-bromobutyrate is no exception. Looking past the long name, you start to wonder: just how risky is this stuff? Safety matters, both for you and the planet. Chemicals like this one turn up in synthetic routes and academic projects, far from public awareness, but not far from real danger.
Sifting Through the Hazards
Tert-butyl 4-bromobutyrate carries a bromine atom on a butyrate backbone. That bromine often spells trouble. Halogenated organics deserve healthy respect. Inhalation can mean coughing, throat pain, or chemical irritation. Skin or eye contact usually results in burning or redness. I’ve been around labs where a small splash led to frantic rinsing at the eyewash station. The MSDS for this compound lists it as harmful if swallowed or inhaled. People might shrug off chemical warnings, figuring gloves and goggles handle the risk. Risk follows familiarity, not the other way around. Friends working prep chemistry often talk about skin rashes after careless glove use. With this compound, short-term exposure may cause irritation, but chronic results could involve organ damage— especially since many halogenated organics sneak through the skin or linger in the air.
The Broader Picture: Disposal and the Environment
Lab doors keep most people out, but everything poured down the drain joins local water systems. Halogenated organics resist breakdown. A few decades back, chlorinated solvents contaminated groundwater. Brominated cousins like tert-butyl 4-bromobutyrate can persist in soil and water. Fish and small aquatic creatures suffer first; these toxins climb up food chains. In my undergrad days, our instructor hammered one lesson home: one careless flush could harm thousands of gallons.
Solutions and Best Practices
For any lab using tert-butyl 4-bromobutyrate, vigilance should be routine. Get your information from the safety data sheet, not from habit or memory. Personal protective equipment—gloves, goggles, and even face shields—makes a real difference. Good discipline means logging every use, knowing where the spill kit is, and cleaning up regularly. To reduce waste, chemists look for alternative reagents if a method allows. Green chemistry isn’t just a buzzword. Chemical suppliers and researchers now sponsor competitions rewarding solvent swaps or safer syntheses that sidestep toxins like brominated esters. In one of my old research groups, we spent weeks testing new protocols that skipped problem chemicals; the learning curve felt steep, but the payoff mattered to everyone working in that space.
As for disposal, hazardous waste contractors offer the safest route. Segregating halogenated waste from the regular trash or sink keeps cross-contamination down. Bottles labeled and tracked reduce surprises for the next person. Local rules vary, but the guiding principle stays simple—better to over-label and over-prepare than to guess.
Staying Informed, Staying Safe
Working with chemicals in the lab means constant learning. Tert-butyl 4-bromobutyrate isn’t among the world’s most famous toxins, but it earns respect for its risks. Just because it works in the flask doesn’t mean it’s safe on your hands, or easy to erase from the ecosystem. Experienced chemists treat each bottle with the wariness it deserves, not just for themselves but for their community and the environment. If the compound bothers you, ask questions, check the research, and never shortcut safety. There’s no downside to being careful; every upside lasts a lifetime.
Why Purity Matters in Chemistry Labs
In research labs, trust in a chemical often comes down to purity. Tert-Butyl 4-Bromobutyrate stands out as a building block in organic synthesis, especially for pharmaceuticals. Contaminants can throw off reactions, create unreliable results, or—worst of all—introduce toxic byproducts. Any chemist who’s wasted a week on a failed synthesis knows the agony of impurity-related setbacks. A high-purity standard can mean the difference between reliable data and wasted time.
Common Specifications Seen in the Market
Most suppliers sell Tert-Butyl 4-Bromobutyrate at a minimum purity of 98%. You’ll sometimes spot a 99% version for really demanding work. That number doesn’t come out of nowhere. GC (Gas Chromatography) or HPLC (High-Performance Liquid Chromatography) usually backs those labels. Reliable suppliers don’t just throw a number on a bottle—they provide a CoA (Certificate of Analysis) showing exactly how the batch performed on testing.
Impurities matter. Some, like unreacted butyric acid derivatives or traces of solvents, might seem harmless at trace levels. In practice, these hidden leftovers can interact in unexpected ways, especially in medicinal development. The tighter the specification, the less chance for downstream headaches.
Looking Deeper Than the Purity Number
A purity number tells part of the story, but chemists need specifics about what’s leftover. Reputable vendors often disclose levels of water, heavy metals, and specific known process impurities. Water, especially, should sit at less than 0.5%. Anything more can cause issues in moisture-sensitive reactions.
Supporting data from reputable suppliers matters. Analytical chemists I’ve worked with always check that a batch matches spectra from NMR and IR, confirming you’re not just getting a cleaner—but also the right—compound.
Practical Reasons for High Purity
Process repeatability relies on pure starting materials. Medicinal chemists can’t risk their trial compound failing due to a mystery impurity that crept in at the start. In the pharmaceutical world, regulatory agencies like the FDA don’t accept excuses. Approval depends on demonstrating that synthesis runs with precisely defined, traceable materials. Low-purity reagents can delay or derail expensive programs.
I’ve seen routine reactions suddenly turn unpredictable due to changes in source material. Maybe a supplier cut corners or switched a solvent in manufacturing without telling anyone. Suddenly, product yields dip, and time-consuming troubleshooting follows. Consistent, documented purity reduces these costly interruptions.
How Chemists Verify What They’re Working With
It’s not enough to take a label at face value. Scientists run their own checks on incoming batches with analytical tools like melting point analysis, GC/MS, or NMR. This extra step has saved labs from months of lost time more than once.
Solutions: Raising the Bar for Purity
Demand for detailed certificates and rigorous testing from suppliers pushes the industry forward. Labs should press for disclosure of all known process impurities and not just a bottom-line purity number. Storing chemicals under the right conditions—cool, dry, away from sunlight—keeps purity intact after delivery.
Takeaway for Everyday Lab Work
Tert-Butyl 4-Bromobutyrate finds its way into countless syntheses, but the story doesn’t end with a percentage printed on a label. Reliable research and product development rely on high-purity reagents, and attention to detail in sourcing, handling, and storage pays off every day on the bench.