Tert-Butyl 5-Bromovalerate: Delving into a Versatile Organic Compound
Historical Development
Chemists have often chased molecular stability and synthetic accessibility, both of which frame much of the history behind tert-butyl 5-bromovalerate. In the early days of ester chemistry, this molecule emerged from the wider effort to add functional handles—like the bromine group—to straightforward esters for more targeted chemical modifications. Legacy works on halogenated esters set the groundwork, showing that introducing a bromine not only tweaks reactivity but also introduces safer alternatives to harsher alkylating conditions. The tert-butyl group, a classic sterically hindered moiety, became popular over time among researchers seeking to modulate reaction outcomes. By the early 2000s, with pharmaceutical and material sciences hungry for specialized intermediates, this compound began popping up in reference libraries as a useful and flexible building block.
Product Overview
Tert-butyl 5-bromovalerate serves as a compact and handy molecule in laboratory and industrial synthesis. As an ester built on a five-carbon backbone, it holds a tert-butyl ester at one end and a bromine atom at the other. This combination appeals to those working on multi-step syntheses. The bromine atom at the terminal carbon reacts efficiently in nucleophilic substitution reactions, allowing the introduction of other groups, while the bulky tert-butyl makes the ester more resistant to hydrolysis, both in transit and storage. Each time I encounter a synthetic bottleneck involving selective alkylation, a reagent like this often presents a practical workaround, preserving both time and yield.
Physical & Chemical Properties
This clear, colorless to pale yellow liquid usually shows a boiling point hovering near 110-112°C at reduced pressure. Density clocks in at around 1.16 g/cm³, giving chemists a keen sense for quantification during liquid transfer. The compound fares dissolvable in common organic solvents such as dichloromethane, chloroform, and ether, but resists mixing with water, thanks to that tert-butyl cap. The molecule itself resists oxidation and respects most lab atmospheres, but the bromine site draws attention from nucleophiles, making it reactive without being fragile. Odor usually reminds me of typical esters; sharp, faintly fruity, and unmistakably synthetic.
Technical Specifications & Labeling
Suppliers typically ship tert-butyl 5-bromovalerate above 97% purity, checked by techniques like NMR and GC-MS. The CAS number used for ordering offers a consistent hook for records, and labels flag both bromo content and the proper hazard statements linked to esters and organobromides. Most bottles list the need for storage under dry, inert conditions to cut down on hydrolysis and side reactions. Flammable liquid pictograms and related wordings signal both the risk and the regulatory compliance needed under global standards such as GHS or REACH.
Preparation Method
Making tert-butyl 5-bromovalerate typically starts from 5-bromovaleric acid. The esterification comes next, usually carried out through a Fisher esterification, where tert-butanol and the acid meet up with an acid catalyst, often sulfuric acid or p-toluenesulfonic acid. Alternatively, direct use of an acid chloride in the presence of tert-butanol and a base, like pyridine, produces the desired ester faster and in higher purity. The reaction keeps up best under controlled temperatures, as overheating can trigger side reactions such as elimination. Chasing yields in my own projects, switching between acid- and base-catalyzed routes always provided a trade-off between speed and overall product cleanliness.
Chemical Reactions & Modifications
The bromine tag on the end of the valerate chain turns this ester into a versatile springboard. Nucleophilic substitution opens the door for amines, thiols, or even carboxylates to join the chain, allowing for a broad array of derivatizations. One-pot synthesis approaches can switch out the bromine for an azide or cyano group with copper catalysis or other specialized conditions. When the tert-butyl ester protection outlives its usefulness, a splash of acid or heat strips it away, yielding a free acid ready to jump into peptide coupling or further functionalization. Modern flow chemistry also takes advantage of tert-butyl 5-bromovalerate, minimizing human handling and improving safety.
Synonyms & Product Names
Literature and suppliers recognize tert-butyl 5-bromovalerate under several names, reflecting its structure: tert-butyl 5-bromopentanoate, 5-bromovaleric acid tert-butyl ester, and the straightforward 5-bromo-pentanoyl tert-butyl ester. International vendors sometimes catalog it as simply TB5BV or TBBPV, streamlining procurement. In my own experience, sorting through chemical inventories gets easier when synonyms match those on the safety data sheets and synthetic protocols.
Safety & Operational Standards
Handling this ester means respecting its dual hazards—a flammable ester and an organobromide with potential to cause skin or respiratory irritation. Personal protective equipment, especially gloves and goggles, stays on throughout use. Fume hoods matter, as the vapor carries both fire and inhalation risks. Disposal protocols treat any halogenated waste with care, tracking it for proper chemical incineration. Laboratory training emphasizes rapid spill response with absorbent material and immediate ventilation. First aid procedures for accidental skin contact and inhalation appear on the SDS and enter regular safety briefings, reflecting the real-world scenarios of daily chemical work.
Application Area
Pharmaceutical synthesis often finds value in tert-butyl 5-bromovalerate’s unique pairing of a reactive bromo and a protected acid group. It serves as an effective intermediate in constructing molecules for antiviral and antifungal research, with its easy conversion to carboxylic acids aiding in medicinal chemistry campaigns. Agrochemical projects also draw on it for quick modifications—pesticide analogs see quicker testing cycles. Polymer chemists leverage the bromine for grafting side-chains onto backbones, especially in specialty materials or adhesives. My experience with this ester often circled back to its time-saving ability during late-stage functionalization, granting flexibility that’s rare in smaller lab settings.
Research & Development
Research teams aiming for greener chemistry have targeted the synthesis and application of tert-butyl 5-bromovalerate. Changing catalysts from harsh acids to enzyme- or solid-supported options trims down waste and opens recyclable routes. Latest publications discuss photoredox modifications, expanding the utility of the brominated site without old-school metal catalysts. Material scientists mix this ester into block copolymer production, exploring improved UV-resistance or tuneable degradation rates. Analytic advances such as real-time in situ NMR allow increasingly precise control of reaction tracking, directly improving batch consistency and safety. The landscape keeps shifting as demand for tailored esters grows in both startups and more traditional process development labs.
Toxicity Research
Studies addressing the toxicity of tert-butyl 5-bromovalerate focus on both acute exposure and potential chronic impacts. As with many organobromides, issues center around irritation and possible long-term organ effects. Recent animal studies point to low oral toxicity but also signal the importance of limiting inhalation and skin exposure due to mild but recurring inflammatory reactions seen after repeated contact. Regulatory reviews underline the need for tight exposure limits, especially since tert-butyl derivatives sometimes show metabolic persistence in soil or water. Lab managers often reference both the compound’s irritation index and its environmental fate, ensuring that accidental release or chronic low-level exposure gets flagged early on.
Future Prospects
Demand for selective and modifiable organic intermediates continues to drive development of compounds like tert-butyl 5-bromovalerate. Opportunities to build more sustainable production lines through renewable feedstocks and solvent-free technologies draw funding and academic effort. Advances in flow chemistry promise improved safety and larger batch scalability, while greener catalysts and in-line process monitoring help drive down costs. Downstream, pharmaceutical and agrochemical trends point towards more personalized molecules—each one benefiting from the reliable reactivity this ester brings to the table. As new data on toxicity and environmental fate surfaces, industry will lean harder toward improved containment and remediation technologies, carving out a role for this molecule not just in classic organic synthesis but in the wider world of sustainable chemical practice.
Looking at Structure, Seeing More Than Letters
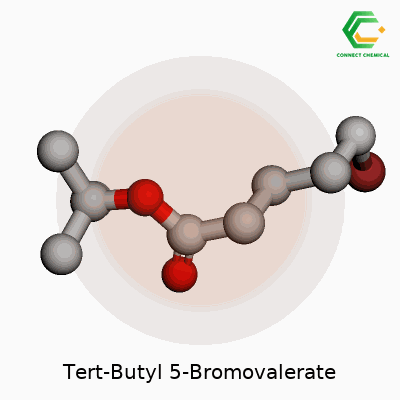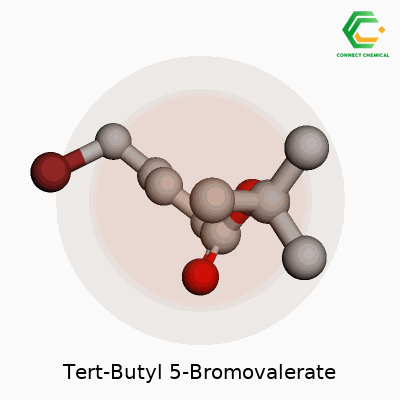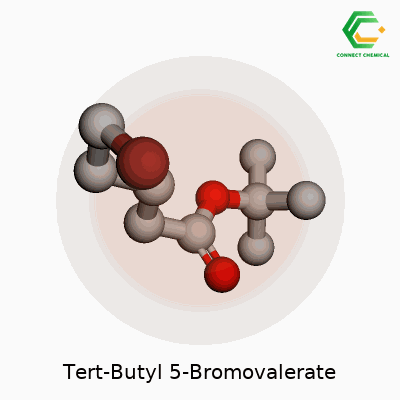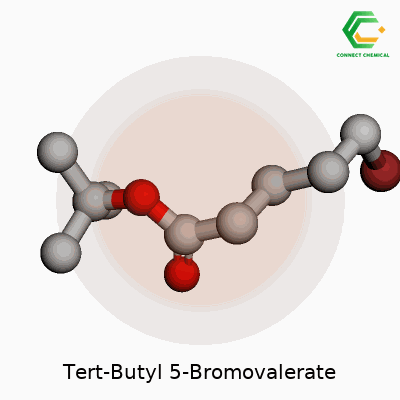
Tert-Butyl 5-Bromovalerate goes by a technical name, but it tells quite a story in the lab. In clear terms, this compound has the chemical formula C9H17BrO2. That breaks down to nine carbons, seventeen hydrogens, one bromine atom, and two oxygens in every molecule. This isn’t just a set of numbers—it reflects a design that lets chemists tweak molecules for a range of uses, from early drug synthesis to explorations in organic chemistry.
The Building Blocks Behind the Formula
To understand this compound, you start by picturing valeric acid, a five-carbon fatty acid. Attach a bromine atom at the fifth carbon, and you get 5-bromovaleric acid. After that, swap the acid’s hydrogen for a tert-butyl group—the bulky “sidecar” that makes the molecule more stable and resistant to certain reactions. Tert-butyl esters like the one here don’t just show up for stability; they let scientists “mask” carboxylic acids so other parts of the molecule react instead. Anyone who has ever run a protection-deprotection sequence in a lab knows the relief when a tert-butyl ester quietly holds everything in place.
Why Specific Formulas Matter in Real Science
It’s easy to gloss over chemical formulas as strings of letters and numbers, but there’s more at stake. Accurate formulas help guarantee the right molecule for the job. Swap one atom and the outcome changes. In pharmaceutical research, putting a bromine atom on the end of valeric acid shapes how drugs interact in the body or attach to other molecules. Medicinal chemists use this route, starting with compounds like tert-butyl 5-bromovalerate, to go after new antibiotics, antiviral agents, or cancer drugs.
Reliable identification keeps work honest. Contamination, mislabeling, or swapping isomers can ruin trials or send research down the wrong path. I remember a project where one mislabeled sample brought weeks of confusion until the error turned up in—ironically—the chemical inventory spreadsheet. Small chains can trip up big advances.
Tackling the Challenges of Working With Organobromines
Synthesis with brominated compounds isn’t just about convenience; safety and environmental impact step into the foreground. Bromine atoms can make molecules highly reactive or even hazardous. Waste disposal calls for strict tracking, and most research labs run regular checks to prevent brominated byproducts from leaking into wastewater. For greener chemistry, researchers hunt for alternative reactions that avoid persistent halogenated waste. Some teams have managed to swap in bio-based esters or find milder reagents that perform the same transformations.
Knowledge-sharing plays a big part, too. When data gets published on reaction yields, side-products, or even failed routes with tert-butyl 5-bromovalerate, it helps the next team dodge old mistakes. Everyone wins when pitfalls get exposed early. Thorough documentation, cross-checking with established databases, and using trusted chemical suppliers keep labs safer and more productive.
Choosing Safe, Reliable Paths Forward
C9H17BrO2 looks simple on paper. For chemists in training and career researchers, that little formula spells out new ways to explore biological function, create materials, or fine-tune pharmaceuticals. Careful handling, accurate reporting, and sustainable disposal methods strengthen every step in making and using compounds like this one. With each experiment, accuracy and responsibility carry as much weight as curiosity or innovation.
The Building Block for Many Chemists
Tert-Butyl 5-Bromovalerate stands out in chemical circles as an intermediate known for flexibility. Put simply, it helps bridge the gap between raw materials and finished compounds. You find chemists at pharmaceutical companies turning to this molecule because it reacts well with a wide range of other chemicals. They’re often looking to produce drugs that rely on custom synthetic routes, and this compound opens up choices in modifying molecular backbones. It’s not about acting as a finished ingredient, but about enabling the production of new drug candidates and specialty materials.
Synthesizing Pharmaceuticals and Fine Chemicals
Much of my own time near research benches has shown me the importance of reliable intermediates. I’ve watched teams use tert-butyl 5-bromovalerate as a base for creating beta-amino acids and various heterocycles. Many antihypertensive drugs have structures that hook up well with this compound’s bromide end. Chemists value the tert-butyl group because they can remove it under controlled conditions—this trick really helps ensure that only certain steps in the synthesis sequence get modified. The product’s chemical stability under mild conditions gives the synthesis process some breathing room, reducing unwanted side reactions.
The World of Agrochemicals
The agricultural industry often borrows tricks from pharmaceutical chemistry. Tert-butyl 5-bromovalerate serves as a go-to intermediate during synthesis of some herbicides and fungicides. Research teams aiming to modify the effectiveness or safety of an agrochemical candidate sometimes swap out raw materials, and this compound shows up in their notebooks a lot. Its five-carbon tail brings flexibility, and the bromide allows for easier substitution reactions. Newer pest-control products, for example, sometimes need changes at the early stage of their creation, and this compound fits that job well.
Academic Research and Method Development
Beyond industry, many graduate students and academic researchers rely on tert-butyl 5-bromovalerate. It acts as a model substrate for testing reaction conditions or exploring new catalytic techniques. I remember seeing it play a role in early cross-coupling experiments, where researchers wanted to see whether new metal catalysts handled bromides effectively. Sometimes, a simple molecular structure like this makes it possible to learn more about how different reactions run, and what tweaks lead to better yields or cleaner results.
Finding Practical Solutions to Sourcing and Waste
Even though this intermediate offers huge potential, sourcing it can occasionally hit a snag. Supply chain hiccups sometimes force buyers to look for alternatives. One solution: local chemical suppliers have started keeping it in stock in response to steady academic and commercial demand. On top of that, waste reduction earns a lot more attention these days. Laboratories have become more aware of the environmental impact tied to halogenated by-products. Chemists often adopt greener solvents and recycling methods to cut down the volume of hazardous waste produced during its use.
The Path Forward
In the end, tert-butyl 5-bromovalerate fills a key position for a range of scientists. The applications keep expanding as new medicines and agrochemicals enter the development pipeline. The industry has room to grow by refining how this intermediate gets produced and disposed. Switching to more sustainable chemistry and supporting domestic production would not only help supply chains, but also protect researchers and the environment long into the future.
Actual Substance or Just a Code?
In a world that celebrates breakthrough molecules and new syntheses, Tert-Butyl 5-Bromovalerate doesn’t show up on glossy magazine covers, but it has its own cult following in the R&D corners of chemistry labs. People use this compound for building more complex molecules, not just as a bench curiosity. Whether it’s pharma, agrochem, or just chemical innovation driving the purchase, buying and using this ester comes down to two things: what it looks like, and how clean it is.
Purity Isn’t Just a Number—It’s Everything
In most catalogs and chemical warehouses, suppliers tend to list Tert-Butyl 5-Bromovalerate at a purity of 95% or above. Someone not involved with synthesis might shrug and think that’s “good enough.” In practice, that’s just the starting line. Researchers who use it as a building block put their trust in these numbers. Cutting corners with purity means headaches—yields drop, byproducts pop up, and uninvited halides or esters might introduce unpleasant surprises during downstream reactions.
The accepted standard at reputable supply houses is >98% for most non-bulk work. Most chemists run a quick NMR or check the supplier’s HPLC data to double-check the lot they receive. In my own graduate work, I never fully trusted certificate values unless I saw a sharp, clean spot on TLC or a single, clean peak on GC. False confidence leads to chasing ghosts through a reaction flask later.
Even small contaminants, especially water, can trigger side reactions—think hydrolysis over time, which means a precious bottle slowly morphs from the desired bromoester to just valeric acid. That’s wasted time and money.
Spotting the Real Thing: Physical Appearance Tells the Story
Anyone who’s peeled open a bottle of Tert-Butyl 5-Bromovalerate will tell you it’s not some mysterious elixir. It flows out either as a clear to pale-yellow liquid. Some suppliers mention a faint odor, often sweet or a touch medicinal, but let’s be honest: the average lab smells so much of ether and solvents that subtle notes rarely stand out.
If you see particles, cloudiness, or an off-putting brown hue, something went wrong—maybe storage, light exposure, or the bottle simply sat open too long in a humid storeroom. Lab techs know the importance of tight seals and desiccators for a reason.
Sometimes the compound crystallizes or thickens a bit near 0°C, not enough to freeze solid but enough to make pipetting annoying. Let it warm a little, and it returns to its usual viscosity. If you’re drawing from an older bottle, check for residue at the bottom—that’s often a sign of slow decomposition.
Making Better Choices, Avoiding Sneaky Surprises
Many researchers feel safe trusting big-brand suppliers, but no system beats a quick quality check in the lab. Even a simple visual inspection goes far—a cloudy ester is worth raising an eyebrow. Most common issues involve lapses in storage, like leaving the cap loose or allowing moisture in, especially in shared workspaces.
A straightforward tip for anyone ordering Tert-Butyl 5-Bromovalerate: ask for a small sample with documentation or buy from a vendor open to sharing analytical data. R&D budgets don’t stretch, and skipping those steps costs more in repeated syntheses than saving a few bucks on a questionable batch.
Final Thought
Clean, clear Tert-Butyl 5-Bromovalerate is more than a label claim—it’s the foundation for reliable, reproducible chemical work. What shows up in the bottle makes all the difference between spending a day troubleshooting or moving on to real discovery.
Why Safe Storage and Handling Matter
Every worker in a chemical lab remembers the first time they saw a vial with a name like Tert-Butyl 5-Bromovalerate. This compound, like many others, won’t usually announce itself with a smell or a color change if something’s off. Complacency is where trouble begins. Fires, health issues, expensive losses—these risks are real if you cut corners with storage and handling.
Storing for Safety
Tert-Butyl 5-Bromovalerate catches fire if left on a hot plate or near sunlight. It breaks down in heat and light, and old hands know clear glass and windows are an invitation for breakdowns. Store this chemical in a tightly sealed container made from non-reactive material, keep it away from direct light, and keep the room cool. Flammable storage cabinets are built for this sort of thing; theft-proof, vented, and made of steel.
Keep incompatible items away. Acids, oxidizers, and bases invite reactions you don’t want. Posting a clear inventory and color-coded labels right on shelves helps new staff find and separate them—but most problems still come from old-fashioned forgetfulness in busy labs.
Chemical companies and research centers track logs on refrigerators and cabinets. These logs show who opened footage, how long items sit before being used, and which staff check expiry dates. There’s more paperwork, yes, but fewer spills and burns.
Handling with Care
Gloves, goggles, and lab coats aren’t suggestions; they’re the basics. One splash or whiff can bring skin irritation or trouble with breathing. During training, most people roll their eyes about the rules until they feel solvent dripping down a sleeve. I remember a co-worker who tried to catch up on a late experiment without checking gloves for holes. The irritation lasted for days and the lesson stuck.
Work under a fume hood. Tiny amounts can evaporate or react in air, creating fumes that build up fast in closed rooms. Open bottles slowly and pour over absorbent pads. Bottles tip easier than you think and once you lose a drop, it’s a race to keep it from spreading on the bench.
Accidents happen even with the best intent. Spill kits should have neutralizing compounds and absorbent pads close by. Even veteran teams practice for spills since panic does more damage than the spill itself.
Training and Accountability
Trust grows with a good system, not promises. New employees watch experienced staff follow the same steps each time, and smart managers schedule regular refreshers—not to fill seats, but to swap stories about close calls. Videos can help, but nothing replaces hands-on practice.
Regular reviews by an outside auditor catch lazy habits before they become incidents. Labs display the latest Material Safety Data Sheets (MSDS) in bright folders or posters for easy checking during a rushed moment.
Looking Ahead
Safety isn’t about being afraid of chemicals. It starts with respect: for the substance, for coworkers, and for your own health. Every time a routine is followed, it preserves trust in the building. In my experience, shortcuts invite headaches and costs bigger than most realize—and those who stay safe end up teaching others by example.
Understanding Safety Concerns
Handling chemicals such as Tert-Butyl 5-Bromovalerate isn’t something anyone takes lightly in shipping or storage. Hazards don't just exist in big industrial settings; plenty of people have learned, sometimes the hard way, that compounds with halogens and esters call for caution. Some folks working with this compound remember strict instructions: one spill and the clean-up involved more than a mop and bucket. That sticks with you.
Regulators treat organobromine chemicals with respect. Tert-Butyl 5-Bromovalerate sits in that group, and the safety data sheets point to irritant effects on skin and eyes, plus risks to aquatic environments. If packaging leaks, the impact could go beyond the lab. Local ground and water get affected, and fish don’t stand much chance once brominated organics reach a stream. Regulations draw from incidents where small mistakes turned into bigger problems.
Shipping Regulations
Moving Tert-Butyl 5-Bromovalerate across state or national borders means meeting strict classification rules. The U.S. Department of Transportation (DOT), for example, has a framework built for flammables, corrosives, toxics, and environmentally hazardous substances. This compound fits the bill as a hazardous material. Shippers must label drums or containers as dangerous goods, complete with United Nations numbers, hazard pictograms, and handling codes. Couriers who deal with chemical shipments rarely miss this step, knowing any missing documentation delays or blocks transport.
The International Air Transport Association (IATA) requires extra paperwork and certified packaging before a chemical like this ever hits an aircraft cargo hold. Agents at airports don’t just accept word-of-mouth or unchecked crates. They run checks for leaky seals, proper cushioning, and labels in the right language. Shipments in glass bottles go inside sealed steel drums, with enough absorbent material to capture leaks. Air travel leaves no room for error. Customs officers at every stop demand proof that shipments meet environmental and safety rules—this isn’t a formality but a real roadblock if overlooked.
Storage and Handling Benchmarks
On arrival, storage rules take over. Chemical warehouses log every drum, inspecting seals before shelving anything near food ingredients, medical supplies, or flammable goods. No one on a loading dock wants to explain why incompatible chemicals shared a shelf. Proper ventilation matters, too. In my own time assisting a storage firm, moving just one drum of organobromine from damp, cramped quarters to a dry, well-aired storeroom made a huge difference in how lingering odors and vapors drifted through the air.
Emergency responders care about this, too. Spill response kits, fire extinguishers, and chemical suits stay close by. Staff go through drills every month, simulating accidents with stand-in fluids to make sure muscle memory clicks in if real emergencies come up.
Solutions to Improve Shipping Safety
Tracking technology now helps. Barcodes and digital records link to shipment history. Staff scan each unit from dispatch to delivery, making it easier to trace the path and react fast if anything goes wrong. Regular training remains crucial. In the last training I attended, folks got hands-on with new absorbents and seals. That kind of experience builds habits better than reading a manual.
Government rules might look complicated, but history shows weak controls invite trouble. Following the right steps, making sure packaging passes inspection, and keeping workers trained turn what could be a risky shipment into just another safe delivery. Those measures matter each time Tert-Butyl 5-Bromovalerate moves from one warehouse to another, across states or overseas.