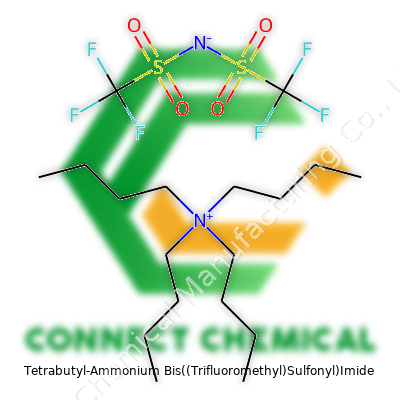
Tetrabutyl-Ammonium Bis((Trifluoromethyl)Sulfonyl)Imide: A Chemist’s Perspective
Historical Development
Tetrabutyl-ammonium bis((trifluoromethyl)sulfonyl)imide — usually called TBATFSI — has a story that starts in the late twentieth century, when chemists started tinkering with ionic liquids to solve stubborn problems in electrochemistry and synthesis. The search for non-volatile, conductive salts pushed folks to rethink the ammonium cation and combine it with new anions. TBATFSI owes a lot to that moment in labs all over the world, where researchers realized that tweaking the anion can make an enormous difference in melting point and solubility. The pairing with bis((trifluoromethyl)sulfonyl)imide, sometimes dubbed TFSI, cracked open the door to safer and more versatile organic salts. Companies in Japan, Europe, and the US built out production for specialized uses across batteries and analytical chemistry, pulling the compound from specialty shelves into advanced manufacturing.
Product Overview
In practice, TBATFSI shows up as a white to off-white powder or sometimes a chunky crystalline solid, depending on humidity and handling. It’s not the world’s cheapest salt, but it has become a go-to electrolyte additive in the research sector. In the lab, the bottle may show up labeled under any one of its chemical names, but most researchers recognize the four butyl groups of the cation and the two strongly electron-withdrawing TFSI anions attached to the core nitrogen. The product ships in sealed glass or heavy-duty plastic, usually under dry atmosphere, since moisture can affect long-term storage and the quality of the product in sensitive applications.
Physical & Chemical Properties
TBATFSI has a formula of C16H36F6N2O4S2. It melts at around 70 to 75 degrees Celsius, but in practice, it often feels sticky before then due to its tendency to absorb moisture. That hygroscopic nature means handling calls for attention: gloves, a good fume hood, and some patience. It dissolves easily in polar aprotic solvents — think acetonitrile, dimethylformamide, or propylene carbonate — which helps in both synthesis and analytical work. The salt brings significant thermal stability, keeping its integrity even above 200 degrees Celsius. Chemically, neither the cation nor the TFSI anion reacts lightly with common bases or acids under standard conditions. This chemical stubbornness suits TBATFSI for harsh reaction mixtures or demanding electrochemical cells.
Technical Specifications & Labeling
Suppliers mark TBATFSI by its purity, ash content, residual water, and sometimes by specific analysis for common metal ions, given that even trace metals wreak havoc in battery or analytical chemistry applications. A typical analytical data sheet lists purity above 98%, water content below 0.5%, and details NMR and IR spectrometry profiles to confirm identity. Safety pictograms focus on eye and skin irritation. CAS numbers and UN identifiers appear on the shipping labels. Researchers look for tight seals and desiccants packed alongside the product, especially for larger quantities, because moisture is the number one killer of salt-based reagents in advanced chemistry.
Preparation Method
Preparation starts by generating the tetrabutylammonium cation, commonly through the reaction of tributylamine with butyl bromide, followed by metathesis with lithium bis(trifluoromethanesulfonyl)imide. The exchange reaction liberates lithium bromide, leaving TBATFSI dissolved in a water-miscible organic phase. Purification involves washing, solvent removal under reduced pressure, and sometimes recrystallization from ethyl acetate or similar solvents to chase out ionic contaminants. Larger manufacturers carefully control parameters so that each lot repeats the properties researchers count on, but on the small scale, clever extraction, drying, and filtration are still the norm.
Chemical Reactions & Modifications
TBATFSI does not participate in dramatic transformations under mild conditions, which earns it praise for stability as an electrolyte salt or phase-transfer catalyst. Those butyl chains barely budge unless hit with strong acids, and the TFSI anion resists most nucleophilic attacks thanks to its electron-poor, fluorinated nature. Still, in the hands of an experienced chemist, analogues can be produced by swapping in other ammonium cations or tweaking the anion’s substituents. Some novel ionic liquids result from such experimentation, lending tailored properties for fuel cells, sensors, and supercapacitors. The salt stands firm in most organic solvents, which helps when running tough reactions under forcing conditions.
Synonyms & Product Names
Some chemists call it Tetrabutylammonium TFSI, others use the full mouthful: N,N,N-tributyl-N-butylammonium bis(trifluoromethylsulfonyl)imide. Catalogs may abbreviate to TBATFSI or flag it by trade names, but in the research community it remains one of a family of widely used organic-inorganic hybrid salts. Accurate naming matters here because closely related salts, like those based on PF6- or BF4-, carry distinct hazards and reactivity. Small mislabeling leads to wasted experiments and compromised results, so Sigma, Alfa, and other suppliers spend extra effort to avoid confusion.
Safety & Operational Standards
Experience teaches that even stable salts like TBATFSI demand respect in the lab. Inhalation of dusts and chronic skin contact both cause irritation, especially for those prone to allergies. Gloves, goggles, and a reliable fume hood minimize direct exposure, while careful waste management reduces risks to lab workers and the environment. SDS (Safety Data Sheet) guidance requires engineering controls and spill containment routines. Outside the lab, facilities working in pilot or full-scale production adopt workplace monitoring, double-containment for bulk storage, and regular training around potential toxic byproducts. TBATFSI does not leach fluoride ions under common conditions, yet strong acids or incineration do present some environmental threats, so downstream treatment of waste solutions gets priority in responsible operations.
Application Area
Most interest comes from electrochemical devices, particularly lithium batteries and supercapacitors, where TBATFSI can replace more hazardous or less stable conducting salts. Battery developers value its broad electrochemical window, low viscosity in solution, and minimal interaction with other battery components. Outside of energy storage, the salt helps analytical chemists as a supporting electrolyte for cyclic voltammetry or ion chromatography, smoothing out background noise and letting signals shine through. Some niche organic syntheses turn to TBATFSI as a phase-transfer catalyst, harnessing its bulky, non-coordinating anion to shepherd stubborn ions from one phase to another. Researchers testing new fuel cell membranes or advanced separation processes rely on salts like this, seeking better balance between ionic mobility and chemical resistance.
Research & Development
Laboratories continue pushing the boundaries for TBATFSI, especially those working at the crossroads of green chemistry and energy technology. Academic groups work to lower preparation costs, shrink the environmental footprint of manufacture, and unlock new reactions where TBATFSI’s stability and solubility open up previously unworkable pathways. Startups hone methods for recycling and recovering these salts, even pulling them out of spent battery electrolytes for refurbishment. While better salts compete in some emerging applications, TBATFSI holds ground thanks to a legacy of safety data, robust supply, and a deep bank of published research. That hard-earned knowledge allows faster troubleshooting, greater reproducibility, and confidence among regulatory bodies.
Toxicity Research
Toxicologists look at both immediate effects—skin, eye, and inhalation hazards—and long-term exposure, especially for those handling bulk quantities. Data shows that acute toxicity remains low compared to heavy metal salts or volatile organics, though fluorinated compounds call for close monitoring in environmental contexts. Ongoing studies track metabolites and breakdown products, particularly since persistent organofluorines sometimes accumulate in aquatic systems. Regulators in Europe and the US now demand more granular risk assessments, guiding both users and producers in safe handling from cradle to grave. For now, gloves, eye protection, and tight ventilation remain the frontline defense in handling the product responsibly.
Future Prospects
Looking ahead, TBATFSI could move farther into mainstream manufacturing as solid-state batteries and green solvents continue their march into commercial use. Persistent R&D around cost-cutting and recycling may open the door to larger scale deployments, lessening the environmental impact and lowering entry barriers for smaller companies. At the same time, careful scrutiny will remain central: sustainability and toxicity studies gain ground in shaping regulatory policy and public trust. The next generation of high-performance, safe, and recyclable ionic compounds may still spring from the backbone built by salts like TBATFSI. Those keeping one eye on the literature and one on the bench stand the best chance to catch new opportunities as they break.
What Drives Chemists to Reach for This Compound?
Chemists can spend years chasing after better solvents. Some options show too much reactivity, others fall short in strength or safety. Tetrabutyl-ammonium bis((trifluoromethyl)sulfonyl)imide, often shortened to TBATFSI, answers a call in the world of ionic liquids and advanced electrochemistry. You won’t see it sitting on the shelf in a high school science lab. It mostly shows up for problems that demand finesse and stability, especially in battery research and custom electrolytes.
Solvents with Staying Power
My time spent supporting lithium battery research showed me how hungry the field is for robust, non-volatile electrolytes. The tiniest development—say, swapping out the salt in your recipe—can mean the difference between a battery that fizzles out in a month and one that sticks around for years. TBATFSI ends up as a favorite because its bulky organic structure, paired with that tough imide group, helps keep ionic liquids stable and easy to handle. In simple terms, this salt blends smoothly into other chemicals that don’t like water, raising the performance of batteries, capacitors, and specialized sensors.
Supporting Clean Tech
It feels good working in a space where real change happens. Renewable energy developers crave dependable energy storage. Flickering, unreliable output does more harm than good. Solid-state and next-generation lithium batteries depend on stable ion carriers. TBATFSI solves more than a chemical puzzle: it helps keep our wind turbines and solar grids running after the sun sets or the breeze dies down. Its gentle nature toward sensitive electrodes and high ionic conductivity puts it in a handful of industry solutions for smart grid storage and electric vehicle projects.
Navigating the Synthetic Chemistry Maze
Beyond batteries and capacitors, TBATFSI gets tapped for its usefulness in organic synthesis. Anyone who spends long afternoons in a lab knows the frustration of a tough reaction that never quite completes. Chemists use TBATFSI as a phase transfer catalyst, especially in environments that punish other salts. This broadens the playbook for building complex molecules or dispensing stubborn ions in custom chemical reactions. A reagent that doesn’t corrode expensive equipment or break down after one cycle saves real time and money.
Challenges and Smart Solutions
It’s not all easy wins—cost, environmental handling, and purity strike as real concerns. Sourcing high-quality TBATFSI can drive up budgets quickly, and waste management isn’t as simple as pouring leftovers down the sink. As researchers, we should encourage manufacturers to refine greener, less toxic production. At the same time, young chemists should know how to recover, recycle, or neutralize spent materials. In the push for eco-friendly energy and electronics, TBATFSI stands as a powerful tool. Success will depend on smart handling, open data sharing, and end-to-end attention to safety through every stage of research and production.
Understanding the Importance of Proper Storage
Managing chemicals in any setting — research labs, factories, even school classrooms — demands real attention. Over the years, I’ve seen what happens when that awareness slips. Chemicals aren’t just numbers and letters scribbled on a label; they're materials that react with humidity, light, air, or even each other. People may feel tempted to relax those rules, but the smallest mistake can bring huge consequences. For example, I once witnessed a simple oversight of keeping a flammable compound near a heat source. The incident never escalated thanks to a quick response, but the lessons stayed with everyone who watched.
Environmental Controls: Keeping Hazards at Bay
Chemicals often respond unpredictably to temperature swings or unexpected light exposure. Take something as common as hydrogen peroxide: leave that in bright light or a warm room, and you’ll notice its punch quickly weakens. Perishables such as enzyme solutions must stay cold in a well-calibrated fridge. If storage happens at room temperature, volatile compounds drift off, while crystals sometimes absorb water from the air — turning powders into sticky messes, causing changes that ruin experiments or worse. Keeping containers firmly sealed, storing them in dry, dark places, and regularly checking expiration dates help avoid these predictable pitfalls.
Proper Labeling Saves Lives
Keeping compounds in tightly closed, labeled containers goes far beyond regulatory checkbox exercises. Confusion in a busy environment leads to cross-contamination or accidental misuse. I’ve worked with colleagues who believed they skipped a step by skipping the labeling. In the panic of emergency, those shortcuts create confusion or, in a worst-case scenario, slow first responders. Stick to large, legible labels listing the chemical name, concentration, and date received. Color-coded systems can be a huge help, signaling—at a glance—if that bottle holds an acid, solvent, or base.
Ventilation and Safe Workspace Design
Many chemicals give off fumes, either from evaporation or slow degradation. Poor ventilation allows for dangerous buildup, sometimes making it hard to know the air has turned toxic. Modern labs typically use fume hoods. In some cases, that’s a luxury; for smaller setups, dedicating a well-ventilated storage area or keeping doors and windows open does a good deal of heavy lifting. I learned to respect the stinging eyes or sharp odors that hint at trouble. Direct exposure can lead to headaches, lung problems, or even fire hazards.
Handling Spills and Emergency Plans
Spills happen, regardless of how careful people are. Only training and clear procedures make a difference. I recommend knowing precisely where absorbent kits and neutralizing powders sit, not only in the storeroom but throughout the workspace. Regular drills embed those reactions into muscle memory. Just as important, containers should open easily without splashing or spreading dust. Users should always wear gloves, eye protection, and possibly masks, especially if powders or volatile liquids are involved.
Looking Forward: Training and Accountability
Ultimately, safe storage and handling depend on ongoing education. At every job I’ve taken, the best programs encourage workers to report near-misses, update safety sheets, and actually practice what’s preached. Investing in high-quality storage cabinets rated for corrosive or flammable chemicals has paid off in peace of mind and saved headaches down the road. Collaborating with chemical suppliers and safety experts also improves compliance and reinforces a culture of trust and responsibility.
Thinking Beyond the Name
Chemicals like Tetrabutyl-Ammonium Bis((Trifluoromethyl)Sulfonyl)Imide—commonly called TBATFSI in labs—aren’t household names, but they do pop up in battery research, electrochemistry, and some niche manufacturing. With a name that long, it usually means serious business. Folks often wonder if it's hazardous or toxic just based on how intimidating it sounds.
Personal Take From the Lab Bench
From my own time working with compounds like TBATFSI, I learned a couple of things fast. Material safety data sheets (SDS) aren’t just paperwork to ignore. I always took a good look before popping open any bottle with “fluoro” or “imide” in the name. TBATFSI itself is a big deal for chemists because it’s stable and does its job reliably as an electrolyte salt in advanced batteries. Those batteries show up in everything from medical devices to sustainable tech. But reliability in performance doesn’t always line up with safety during handling.
Hazards Found Down in the Details
Let’s break this down: TBATFSI doesn’t catch fire easily, won’t explode, and won’t eat through glassware. But the trifluoromethylsulfonyl parts of the molecule raise red flags for good reason. Perfluorinated compounds stick around in the body and environment. Inhalation, skin contact, or accidental ingestion—none of these are ideal with TBATFSI. The compound irritates the skin and eyes, and if it gets in your mouth or lungs, it can do some real harm.
Research from organizations like the European Chemicals Agency labels it as irritating and warns about acting with care. In one peer-reviewed study, I noticed researchers wore full gloves, lab coats, and worked only in ventilated fume hoods, showing that nobody in the know takes shortcuts. Chronic exposure hasn’t been fully studied, which worries people working in these research spaces. Compared to sodium chloride or baking soda, TBATFSI belongs in a locked drawer, not a kitchen shelf.
Environmental Persistence Isn’t Forgotten
Industry uses TBATFSI because of its thermal and chemical stability. These qualities make it tough to clean up if it gets dumped down the drain. PFAS-related compounds—sometimes called “forever chemicals”—don’t really break down in nature, and TBATFSI has a similar backbone. A lot of environmental chemists are now pushing for green chemistry alternatives, worried that accidental spills or improper disposal might sneak this stuff into waterways or the food chain.
Pushing for Smarter Solutions
From my point of view, clamping down on risk starts with respect and knowledge. You’ve got to keep TBATFSI and similar compounds away from untrained hands. That means up-to-date training, clear labeling, and access to spill kits and first aid. Research labs should review waste protocols and explore less persistent substitutes. Regulatory folks keep looking for more data because no one likes surprises 10 years down the road.Making new rules isn't easy, but open lines between researchers, industry, and policy-makers help move the ball forward. Better data sharing and encouraging companies to test new alternatives go a long way.
Hazard or not, TBATFSI deserves careful treatment. It’s not about fear—just about handling tomorrow’s tools with today’s smarts.
Pride in the Details: Why Purity Matters
Years of working in labs and warehouses have shown me that purity isn’t just a percentage on a label. It’s the difference between successful experiments and wasted resources. A high-purity product sets clear expectations. It shows respect for process controls, skill in handling raw materials, and real care for users down the line. When a chemist picks up a reagent labeled “99.5% pure” and runs a reaction, there’s trust built into every decimal point. For food or pharmaceutical workers, certainty about purity can mean safety and trust for entire communities.
In the chemical industry, purity rarely happens by accident. Whether you’re growing crystals or blending powders, someone has to manage potential contaminants at every step: extraction, isolation, packaging, shipping. Each process can introduce unwanted byproducts—tiny but troublesome. Impurities can trigger unwanted reactions, cause off-flavors, or even make a batch completely useless. Stories are everywhere about times a minor contaminant—maybe residual solvent or trace metals—undermined a week’s hard work. These experiences leave a mark and shape thoughtful habits.
Physical Appearance: Not Just About Looks
Anyone who’s cracked open a fresh bag of white powder or poured a new chemical solution can tell you—appearances speak volumes. Color, texture, smell, and how a product pours out often give early hints about quality. Discoloration means oxidation or contamination. Unusual clumping can reveal moisture problems before you get anywhere near a microscope. Small crystalline particles have a certain look and “feel” in the hand, and seasoned staff notice changes within seconds.
I once worked in a compounding facility where a batch of granules looked off-white instead of bright yellow. The team caught the issue before shipment, traced it back to a supplier, and saved weeks of hassle for the customer. No analyst waits for only the fancy instruments to spot a problem. The process starts with sharp eyes and an honest sense of what’s normal. Consistent appearance usually signals a tightly controlled process. Unexpected changes force good teams to investigate, document, and respond quickly.
Looking Deeper: Facts and Solutions
According to the U.S. Pharmacopeia guidelines, small visual checks catch contaminants—dust, glass, or stray fibers—that purity tests alone might miss. In food processing, guidelines from the FDA focus not just on chemical analysis but also on checks for color and texture, especially in bulk shipments. Poor appearance can signal a breakdown in the chain of custody—maybe someone stored a barrel too long or let in too much moisture.
Keeping purity high and appearance steady means putting real effort into supplier audits, standardized storage, and careful handling. I’ve seen companies invest in extra filtration and air-controlled packaging lines, not because regulations told them to—but because too many costly mistakes stack up otherwise. Reliability can boost confidence for every technician and customer, creating real value chain-wide.
Building Trust in Everyday Work
Taking purity and appearance seriously pays off not just for labs and manufacturers, but for the people who rely on finished products every day. Whether it’s vitamins for families or compounds for next-generation batteries, clarity and confidence start by refusing to settle for “close enough.” My experience tells me that keeping eyes open, asking hard questions, and honoring simple details can prevent big problems before they start.
Looking Closer at Potential
Batteries run the world these days. Laptops, phones, cars, even power grids can't go far without innovation in battery tech. As someone who spent years tinkering with homemade solar panels and cobbling together battery packs in my garage, I've learned to think about materials in simple terms: does it boost performance, stay stable under real-world use, and offer a better deal for both nature and wallet?
What the Compound Brings to the Table
Most compounds up for battery duty get tested for three basic things: can it move ions well, keep its cool in tough conditions, and last more than a handful of charging cycles. Materials like lithium cobalt oxide stuck around because they ticked a lot of boxes decades ago, but new compounds keep showing up to challenge the old ways.
Let’s take a fresh candidate. Is it a transition metal oxide, a novel silicon-carbon hybrid, or maybe even an organic salt? Every compound brings its own strengths and headaches. Solid state chemists keep a close watch for new combinations that might open up higher voltages, tougher electrodes, or safer operations. Sometimes lab excitement meets disappointment in scale-up, but persistence keeps the breakthroughs coming.
Performance Beyond the Lab
Lab results look great on paper, but field reality sets a higher bar. Many promising compounds suffer from sluggish charging below room temperature or unpredictable expansion during charging. My old mentor at the university used a battery analogy: “it’s like putting a high-octane fuel in an engine built for diesel—it won’t end well.” Any compound fit for batteries needs to show up to work every day, under every condition most people encounter, not just under perfect testing circumstances.
Cycle life matters more than most admit. A battery might start strong, but drop off after fifty, a hundred, or five hundred cycles. Some materials survive just fine in button cells but crack apart in pouch cells that run cars or grid storage. These details keep folks in the battery field up late, trying to crack what seems like simple chemistry turned stubborn in practice.
Environmental and Economic Costs
Some of the most exciting research focuses on using materials that don’t rely on rare or expensive elements. Cobalt sparked global worries—human rights, toxic mining, and unstable supply chains drive companies to hunt for alternatives. If this compound comes from more common resources or runs cleaner in recycling, that’s a genuine step forward. Batteries need to last, but we shouldn’t have to trash the planet just moving electrons around.
Pushing the Limits: The Path Forward
Materials science never stops. A compound that looks average today can become the next sensation with a tweak or a smart processing trick. Electrolyte additives, nano-coatings, or using a compound as a mix-in rather than the star can all give surprising results. Companies like CATL and Northvolt keep diving into new mixes because something better might be just around the corner.
Government funding and public-private partnerships speed up the research pathway. Labs share data, push open access, and compare test results so snake oil stays out and real breakthroughs stand out. Manufacturers want to know if a compound will shave pennies off production and boost safety ratings—a tough balance, but achievable with persistence.
Battery history is packed with failed contenders, but every once in a while, the right new compound goes from curiosity to common sense. All it takes is a material that handles the abuse, charges up quick, and sticks around for years—without wrecking the world around it.