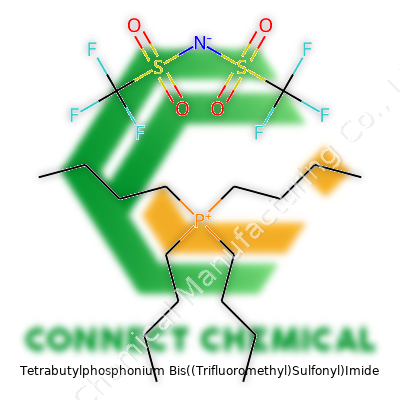
Tetrabutylphosphonium Bis((Trifluoromethyl)Sulfonyl)Imide: A Practical Commentary
Historical Development
Back in the late 1990s and early 2000s, scientists started unlocking the potential of ionic liquids for green chemistry. Tetrabutylphosphonium bis((trifluoromethyl)sulfonyl)imide grew out of this push for solvents that wouldn’t evaporate away or catch fire when bumped into a Bunsen burner. Research labs in Europe and Asia churned out a handful of tailored phosphonium and imidazolium compounds, chasing higher chemical and thermal stability. Phosphonium-based ionic liquids stood out for shrugging off water and stubbornly hanging onto their unique physical properties under heat and pressure. After a decade of scattered work, companies started selling high-purity samples for researchers, shifting the use of the compound from academic curiosity to a workhorse for electrolytes and catalysis.
Product Overview
Tetrabutylphosphonium bis((trifluoromethyl)sulfonyl)imide comes as a pale-colored, sometimes slightly oily, salt. Its chemical formula is C20H40F6N2O4PS2, with a molecular weight hovering around 610 grams per mole. In the lab, it usually shows up in bottles labeled with the shorthand “TBP-TFSI.” Most suppliers pack it as a tightly sealed powder, though its slick nature sometimes makes it look borderline liquid, depending on purity. It dissolves well in organic solvents, showing the flexibility chemists want for tricky tasks like drying or preparing custom electrolytes for electrochemical devices.
Physical & Chemical Properties
Run your fingers over the technical sheet, and you’ll spot a boiling point that’s not listed because decomposition typically kicks in before it vaporizes. TBP-TFSI melts somewhere between 35 and 45°C, a range most labs can handle without special equipment. It carries a faint odor reminiscent of classic sulfur compounds, coupled with the tell-tale tang of fluorinated chemistry. The ionic nature makes it conduct electricity well, a property researchers mine for in battery and supercapacitor work. Chemically, it resists both strong acids and bases, hangs tough in the face of heat, and stays stable for months if it’s kept away from strong oxidizers.
Technical Specifications & Labeling
Suppliers label each bottle of TBP-TFSI with the batch number, molecular weight, purity (95% and higher in most cases), synonynms such as “TBP-TFSI” or “tetrabutylphosphonium bis(trifluoromethylsulfonyl)imide,” and storage recommendations (cool, dry, tight-lidded container out of strong light). Safety warnings include details from the latest European Chemicals Agency reports—skin and eye irritation at higher concentrations, potential aquatic toxicity, and advice to wear gloves, goggles, and use inside a fume hood. Shipping codes mark it as hazardous in bulk, but small lots often clear customs without much fuss since the risks mainly affect workers regularly exposed to the powder or concentrate.
Preparation Method
The synthesis involves reacting tetrabutylphosphonium chloride with lithium bis(trifluoromethylsulfonyl)imide in a solvent like acetonitrile or dichloromethane. After shaking and stirring this mixture, the new salt separates into layers. Chemists use water washes to remove residual lithium salts and organic residues. Drying under vacuum grabs the last of the solvent. In industrial settings, continuous flow methods speed up this process while minimizing waste. For researchers, crystallization from anhydrous solvents polishes up the product, ensuring little to no water or chlorine remain. Every batch gets checked with spectroscopic tools—mass spectrometry and nuclear magnetic resonance—to confirm the structure and spot any hint of contamination.
Chemical Reactions & Modifications
Though it handles itself well against many aggressive chemicals, TBP-TFSI occasionally meets its match in strong nucleophiles that punch through the phosphonium center, breaking down the molecule. Versatile in electrochemistry, it serves as a support salt in non-aqueous electrolytes and can stabilize reactive species longer than most other ionic liquid anions. Some labs tweak the substituents on the phosphonium ion to improve solubility or change melting points, chasing better performance in applications like batteries or carbon dioxide capture. Side reactions are rare, but controlling the environment—scrupulously dry glassware and clean techniques—keeps surprises to a minimum.
Synonyms & Product Names
Across catalogs and safety data sheets, this compound pops up as TBP-TFSI, tetrabutylphosphonium bis(trifluoromethylsulfonyl)imide, or tetrabutylphosphonium bistriflimide. Some older texts call the anion “NTf2” or simply “TFSI,” a nod to its use across different cation pairings. Researchers buying from chemical suppliers should double-check registry numbers, as the compound’s many aliases can be the source of confusion, especially in international markets where translation or local regulation adds another layer of ambiguity.
Safety & Operational Standards
Direct contact with skin leaves a nasty, burning feeling, and airborne dust forces sneezing and coughing in even the well-ventilated lab. Exposure controls usually mean standard nitrile gloves, splash-proof goggles, and keeping the salt inside a fume hood or well-sealed vial. TBP-TFSI encourages respect during weighing and transfer. Workers need training on proper cleanup and how to spot symptoms of overexposure like headache or skin rash. In case of spills, bentonite clay or commercial absorbents pick up residue, followed by disposal as hazardous organic waste—most municipal water engineers shudder at the thought of ionic liquids in groundwater. Storage guidelines call for a stable, cool spot far from acids or strong bases, marked with clear hazard signage.
Application Area
This compound left its mark in the world of batteries first, allowing energy researchers to sidestep volatile organic solvents. TBP-TFSI acts as both electrolyte component and stabilizer in lithium-ion and redox-flow batteries—industries always hungry for longer cycle life and better safety. Electroplating outfits reach for it when working with sensitive metals or trying to smooth out deposit layers in complex alloys. Analytical chemists leverage it for its non-flammable nature in harsh separation techniques, and it makes cameo appearances in carbon dioxide absorption and filtering technology. Over the past decade, demand expanded for TBP-TFSI in organic synthesis, especially when a process needs non-traditional, water-free reaction media.
Research & Development
University and industrial labs team up to push this ionic liquid into new spaces. Material scientists probe its behavior in nozzle-printed electrolytes or blend it with polymers for flexible electronics. There’s a constant race to lower the cost of production without sacrificing purity, since impurities often ruin battery performance or poison catalysts. Some researchers chase better recyclability by engineering ionic liquids that break down harmlessly under mild conditions, closing the loop on chemical waste. Conferences buzz with poster sessions showing real data for conductivity or decomposition temperature, reflecting the collective energy behind pushing TBP-TFSI further into practical domains.
Toxicity Research
The rise of ionic liquids brought environmental toxicologists into the picture. TBP-TFSI, with its fluorinated sulfonyl group, sometimes lingers in soil and water longer than old-school solvents. Bioassays track how the compound moves through aquatic life and breaks down over time. Studies suggest moderate toxicity to fish and algae at high concentrations, enough to warrant care in disposal. Chronic exposure in rodent models hasn’t shown dramatic long-term effects at low doses, but researchers caution that more work remains, especially as ionic liquids spread to industrial scales. Companies and universities work together to map the fate of these compounds in real-world conditions, helping regulatory agencies write smarter, targeted safety guidance.
Future Prospects
Nobody’s putting the brakes on TBP-TFSI soon. New battery chemistries, solvents for polymer recycling, and electrochromic devices all create fresh demand. Scientists keep scanning for ways to strip out the fluorine or tweak the structure for greater biodegradability. Regulatory pressure may someday drive development of alternatives with less environmental baggage, but for now, TBP-TFSI stands at the crossroad of efficiency, safety, and performance. Better public data on long-term health impacts and environmental footprints will shape its next decade—alongside steady improvements in manufacturing processes and waste treatment.
A Closer Look at the Unusual
Once in a while, I come across a chemical name so long that it takes more than one breath to get through it: Tetrabutylphosphonium Bis((Trifluoromethyl)Sulfonyl)Imide. Those who spend their days in labs or industry probably see it on orders or technical sheets. For most people, though, it's just a head-scratcher. So, what does this stuff do, and why do some folks consider it a big deal?
Why It Catches Scientists’ Attention
There’s no escaping the fact that chemistry evolves with society’s needs. This salt has turned into a bit of a star because of its unusual mix of stability, very low volatility, and sheer usefulness. You won’t find it sitting on grocery store shelves. Look in research labs, electronics factories, and advanced energy pilot plants, and it’s another story.
It belongs to a group called “ionic liquids,” which have a liquid form at room temperature. That unlocked a host of possibilities that traditional water- or oil-based solvents couldn’t match. People realized these liquids could be almost tailor-made for new kinds of chemical reactions, high-tech processes, and even greener solutions for waste or pollution. I remember the first time I heard about ionic liquids used to swap out toxic solvents, and it spoke to something personal—working around classic chemical nasties isn’t high on my list.
How It’s Used Right Now
Labs turn to Tetrabutylphosphonium Bis((Trifluoromethyl)Sulfonyl)Imide when processes must stay stable, dry, or non-flammable—situations where old-school options break down or get dangerous. In batteries, manufacturers are always searching for electrolytes that hold up under stress from cycling and charging. This compound handles heat and voltage swings better than many alternatives, and doesn’t catch fire as easily as standard battery fluids. That matters to folks working on safer electric cars, grid storage, and personal devices.
Electronics isn’t just about shiny gadgets; it rests on processes that push the limits of precision and cleanliness. Semiconductor makers need substances that act both as solvents and as electrical insulators. Ionic liquids like this one let researchers clean, coat, and etch surfaces more reliably, supporting the chips that run everything from smartphones to satellites.
Building a Case for a Lower-Impact Future
One of the first things I notice about this material is the growing pile of studies on its environmental profile. Unlike lots of older chemicals, it doesn’t just float away into the air so you end up breathing it. That said, disposal isn’t automatic: it can stick around in water or soil, and toxicology is always a concern. Responsible handling stands as a baseline if we want to keep making progress with fewer side effects.
Industry can’t simply wait for the perfect material. We use what works, then keep pushing for better. More recycling, tighter regulations, and improved testing should stand alongside innovation. I’ve seen colleagues in academic settings push for more disclosure and better waste tracking anytime new chemicals show promise. The move toward more open reporting has made it harder for bad practices or hidden risks to linger—and that fits with public trust in technology.
Looking Ahead
People tend to see chemistry as distant or unapproachable, but it shapes how we tackle some of the biggest issues—energy, waste, product safety. Tetrabutylphosphonium Bis((Trifluoromethyl)Sulfonyl)Imide won’t become a household name, but it marks another step in the search for smarter, safer tools. As regulators, industry, and scientists pay closer attention, responsible choices matter even more—because a great material only helps if it’s handled right from research through real-world use.
Understanding Molecular Patterns
Most folks might look at a compound name and shrug. But knowing what those little letters and lines mean opens up a new world in science, medicine, and environmental safety. As someone who’s worked with both students in a classroom and colleagues in the lab, I’ve seen what happens when people truly “see” a structure for the first time. That moment matters, because a compound’s structure shapes everything — from the way it reacts to its health effects.
Take glucose as a simple example. Its formula, C6H12O6, looks like dry code at first glance. But draw it out and each bond tells a story: six carbon atoms stitched together, with hydrogens and oxygens sticking off like bristles. Arrange those atoms differently and that glucose suddenly becomes fructose. The taste in your mouth, your blood sugar jump, and even what microbes do with it all shift. The difference is a matter of connections, not just the ingredients.
Health and Industry Ride on Structure
You can’t talk about chemical structure without talking about risk. Some compounds look harmless on paper but turn toxic in the body. That’s what keeps chemists and regulators awake at night. The structure determines if something slips past a membrane or sticks around in the bloodstream. The infamous case of polychlorinated biphenyls (PCBs) drives home the point. They are just rings of carbon with attached chlorine atoms. Yet, their shape lets them hang around in fat tissues for decades, causing harm long after production stopped.
Misreading a formula or structure isn’t just a classroom error. It can snowball into real problems — from faulty industrial processes to drug mishaps. Years ago, thalidomide, a sedative, carried a hidden twist in its structure. One mirror-image version soothed morning sickness. The other caused birth defects. One slight shift in chemical shape, and families dealt with tragic consequences. That lesson sticks, sounding a warning any time a new formula pops up in safety testing.
Translating the Science
It’s tempting for those deep in chemistry to toss around complicated diagrams and expect everyone else to catch up. That attitude builds walls. People working in healthcare, food processing, or even home repair all bump into new chemicals. They deserve explanations that break down what those diagrams mean for safety, performance, or compliance.
Good science sticks when it stays accessible. I remember walking through a community garden years ago, where a neighbor asked why composting worked. We knelt, poked at decomposing scraps, and started sketching carbon, hydrogen, and oxygen links with sticks in the dirt. Suddenly, biochemistry made sense — not because the formulas changed, but because someone took time to connect the dots to real life.
Better Communication Means Fewer Surprises
Common language around chemical structures and formulas isn’t just about teaching; it builds trust. When emergencies happen — a chemical spill, a contaminated shipment, or a mystery illness — clear charts and candid talk can calm fears and save time. Fact-based communication helps, backed by visual resources and simple analogies.
As new materials pop up, from greener cleaners to next-generation medicines, the demand for honest, practical explanations grows. The information tied to these structures holds power — not to frighten, but to inform and give people control over their choices. Understanding this area isn’t optional for anyone who wants a safer, healthier future.
Down-to-Earth Advice on Handling Hazardous Products
Growing up around my father’s garage, I learned early that safety instructions come written for a reason—even though, like many, I used to shrug off the warnings. It often takes a mishap, like a splash of solvent landing on the skin or, worse, fumes creating a burning sensation in your throat, to realize that a product’s risks shouldn’t be underestimated. In the world of chemicals, cleaning supplies, gardening products, or construction materials, respect for instructions can make the difference between a routine task and a health hazard.
Why Labels Matter More Than We Think
Manufacturers do not throw together labels out of thin air. Federal agencies like OSHA, the EPA, and global organizations such as the United Nations require standards for labeling and content warnings for good reason. Studies from the Centers for Disease Control and Prevention show that accidental exposure to household chemicals causes thousands of emergency room visits every year. Each pictogram, warning, and signal word exists because someone, somewhere, ended up in a hospital after ignoring basic safety rules.
Common Sense Steps That Save Skin and Lungs
Before cracking open any product, I always check the lid for safety advice—things like “use in well-ventilated area” or “wear eye protection.” These aren't overkill. Good ventilation cuts the odds of inhaling fumes that can trigger asthma attacks or worse. Once, I watched a coworker cough for days after using spray adhesive in a small, closed room.
Gloves and safety glasses come in handy far more often than people want to admit. Chemical burns can develop from something as familiar as bleach. Gloves are inexpensive and avoid trips to the clinic. A simple pair of goggles stops caustic splashes before they reach your eyes.
Washing hands after handling any product sounds basic, yet many folks rush through cleanup. Chemicals can hang around on skin and settle on doorknobs, light switches, or even food. My family keeps a box of disposable gloves by the garage door. This tiny step cuts risk for the whole household.
Safe Storage Routines Build Good Habits
Products should not sit within reach of kids or pets—locked cabinets protect more than curious toddlers. In my neighborhood, a dog once ended up at the vet for licking up spilled antifreeze, which can taste sweet but turns deadly fast.
Storing products in their original containers helps avoid confusion. Pouring liquids into unlabeled bottles courts disaster. Many cases of poisoning stem from children drinking from mystery bottles. Labels carry crucial treatment instructions for doctors and paramedics if a problem arises.
Steps for Safer Use at Work and Home
- Read every part of the label before opening anything unfamiliar
- Open windows or use fans to improve airflow, even for products that seem mild
- Wear simple safety gear—gloves, goggles, even a dust mask for powders or sprays
- Keep children and pets away until every surface has dried or settled
- Store leftovers high up or in locked cabinets—not in food or drink containers
- Dispose of unused material following instructions—not by pouring down the drain
Hands-On Tips Lead to Everyday Safety
No label can predict every risk. Being mindful, looking for proven advice, and sharing tips with others—these steps protect people at home and work. Every caution comes from a lesson someone learned the hard way. Safety doesn’t slow things down—it just lets everyone get back to life without needing a doctor’s visit.
Understanding the Chemical
Tetrabutylphosphonium bis((trifluoromethyl)sulfonyl)imide goes by the mouthful of a name, but many know it as a high-performance ionic liquid. Folks in the chemical industry tap it for its stability, non-volatility, and handy solubility. These qualities mean it sees real action in high-tech batteries, advanced catalysis, and specialty synthesis. With properties like these, safe handling and storage deserve more than a casual approach.
Respect the Real Hazards
This substance shows a strong resistance to water and oxygen, which can sound reassuring. Some might shrug at it—yet hazards don’t disappear just because a material doesn’t ignite or explode quickly. Liquid spills or vapors might slip by without drama, but chronic exposure or improper containment breeds trouble. Skin and eye irritation, along with environmental harm, remain real risks if rules slip.
Skip Shortcuts—Contain with Confidence
Store the chemical in double-sealed, tightly closed glass or high-quality plastic bottles. No one wants to scramble for a cleanup because the cap failed. It rarely leaks out, but sharp eyes should scan for even a small crack around the lid. Fluctuations in temperature won’t usually break it down, but keeping it at consistent, cool room temperature gives real peace of mind. Sunlight isn’t a friend—dark cabinets matter, as some chemical structures break down with excess light.
Don’t Mix—Keep Chemicals Apart
Too often, chemicals pile up on the same shelf. A spill or hint of moisture can start a mess—especially with reactive metals, strong oxidizers, or acids. This ionic liquid won’t burn with a spark, but it can start unwanted reactions with more aggressive neighbors. Pick a spot away from acids, oxidizers, and anything that could share its shelf in the wrong way. Invest in chemical storage cabinets, especially if your lab works with both acids and ionic liquids.
Avoid Accidental Spills
Spills in the lab rarely start with a dramatic splash. Small leaks or careless handling leave stains and contamination without fanfare. Every time you touch the bottle—after pouring, wiping dust, or checking inventory—look for residue and seal it again. Good labs use secondary containment trays, so even if a bottle tips, the mess stays in one spot. It sounds like extra work, but a single oversight can lock up an entire research operation for cleaning and decontamination.
Ventilation and Safety Protocols
Keep good airflow wherever this compound lives. Even stable ionic liquids sometimes release trace vapors you can’t see or smell. Decent ventilation protects anyone on the premises, especially during drum opening or bulk transfer. Safety goggles and gloves aren’t negotiable; the stuff is gentle by comparison to some acids, but gentle isn’t the same as harmless. Every reputable supplier echoes this point in safety data sheets—ignore it, and accidents follow.
Label and Track Inventory Like Your Grants Depend on It
No one likes paperwork, but every academic and industrial lab should treat each bottle as if it’s invaluable. Label the container clearly with the full chemical name, date received, date opened, and the owner’s name. Use a digital inventory to track usage—overlooked containers sitting for years can decay, making removal hard and hazardous. Responsible inventory cuts costs, improves safety, and helps when regulators visit.
Building Safer Habits
Every time I’ve seen a careless storage practice, it’s come down to habit, not intent. Safe chemical storage keeps researchers healthy and operations humming. With a few minutes of work—checking seals, tracking inventory, and choosing the right storage spot—problems shrink, work flows smoother, and labs earn trust from funders and regulators. No one wins by betting on luck. Sound habits keep progress running and people safe.
The Daily Impact of Different Product Grades
Most people rarely notice the “purity” or “grade” of ingredients in their daily lives. Next time you grab a bottle of over-the-counter painkillers or pick up a baking ingredient, take a look in the corner—somewhere on the label or the batch sheet, you’ll see a grade or a purity percentage. What’s behind those numbers? These small details can shape the quality, safety, and price of so many products we use. In my years as a science writer, checking the label has become second nature. The story of purity or grade is really about how carefully a product has been made and where it should be used.
Different Grades for Different Uses
Manufacturers often divide products by purity levels because not every use asks for the same standard. Take table salt, for example. What's stocked on grocery shelves is “food grade,” free from harmful contaminants and safe to eat. Lab technologists reach for “analytical grade” sodium chloride, with even fewer impurities—critical for chemical reactions that can’t be thrown off by a trace of something extra. Industries like electronics or medicine sometimes specify “ultra-pure” or “pharmaceutical grade,” keeping impurities at a minimum. These standards protect both the end product and the people who use them.
Purity isn’t just a marketing claim. It’s tested—again and again. The United States Pharmacopeia (USP) checks pharmaceuticals. Organizations like ISO set benchmarks across industries. These rules tighten as the stakes get higher. Consider injectable medications: even a minuscule contaminant can risk lives. Companies step up their purification and screening to make sure that never happens.
Hidden Costs and Compromises
Ask shoppers about “premium” products and many will say the difference is in the price tag or the packaging. For suppliers, pushing for higher purity adds real costs. Every layer of purification means more labor, better equipment, longer wait times, and testing, all adding up. Here’s the catch: not every application benefits from the purest grade. Using analytical-grade chemicals to mop the floor makes no sense, wastes money, and possibly loads the environment with unnecessary chemicals.
On the flip side, cutting corners on purity can lead to serious problems. In my old high school chemistry lab, our teacher refused to let us cut cheap “industrial” chemicals with experiments requiring precision. She’d seen failed outcomes and chalked it up to someone using bargain-grade supplies. In pharmaceuticals and food, the consequences grow bigger—health risks, product recalls, and even lawsuits.
How Consumers and Companies Can Make Better Choices
The answer lies in education and clearer labeling. Making purity levels and grades obvious on every product helps buyers know what’s up to the job. For companies, investing in rigorous quality checks (think batch testing, clear documentation, and standardized sourcing) not only builds trust but also sidesteps bigger problems. Sometimes, regulations force the issue, keeping everyone honest—especially in food and drug sectors. Beyond rules, it comes down to ethics: would you trust your dinner or your child’s medicine to something questionable?
I’ve learned that quality has a long memory. Cutting corners on something simple like a base ingredient can ripple through the supply chain, denting reputations and costing far more than a little extra spent on getting it right. Choosing the right purity or grade is really a shortcut to building trust day after day.