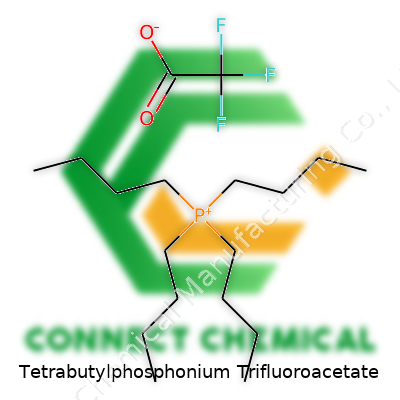
Tetrabutylphosphonium Trifluoroacetate: More Than Just Another Ionic Liquid
Historical Development
Tetrabutylphosphonium trifluoroacetate reflects the steady march of ionic liquid chemistry from its early academic roots to a place in industry. Back in the 1970s and ‘80s, researchers focused mostly on imidazolium and pyridinium-based salts. The shift toward phosphonium ionic liquids picked up in the early 2000s, driven by the search for solvents that outperform legacy organic compounds. This compound stands out for more than its niche applications—it signals a broadening of the field beyond the clichéd “designer solvent” trope. By the mid-2010s, reports started to highlight the value of such salts in catalysis and separations, with Tetrabutylphosphonium trifluoroacetate seen as a prime candidate for both its chemical stability and unique solubility patterns.
Product Overview
Many in the lab might see Tetrabutylphosphonium trifluoroacetate as “just another salt,” but experience quickly proves how differently it behaves. Unlike sodium chloride or even more familiar ionic liquids, the bulky phosphonium cation disrupts regular ionic packing, driving down its melting point and making the liquid state accessible over wider temperature ranges. The trifluoroacetate anion adds to its appeal; it brings strong electron-withdrawing character, which tames nucleophilic reactions, and sheds light on why researchers reach for this salt during especially touchy synthetic procedures.
Physical & Chemical Properties
Anyone who’s handled this compound knows its appearance: colorless, highly viscous, with a faint chemical scent reminiscent of warm plastic. It flows sluggishly at room temperature, clocking in a density of roughly 1.05 g/cm³. Unlike many ionic liquids, it resists water uptake for hours, hinting at robust moisture tolerance that suggests its value in open-system work. Its melting point lags far behind those of conventional organic salts, hovering well below ambient temperature. Thermal stability impresses for such a “soft” ionic material—the stuff rarely decomposes below 250°C. Most reactions won’t shift this structure, which owes much to the shielding effect from the tert-butyl arms of the phosphonium core. Strong acids or bases tend to leave the salt untouched, except under extremes not encountered in daily lab life.
Technical Specifications & Labeling
Product sheets usually list purity upwards of 98% for research grades, with strict moisture control—water levels stay below 500 ppm if handled correctly. Labels often highlight the sensitivity of the salt to UV degradation, a quirk of the trifluoroacetate moiety. Storage advice reads like that for many ionic liquids: keep it cool, shielded from light, desiccated if possible. Typical containers include amber glass bottles with PTFE-lined caps, helping users avoid unexpected contamination. For industrial quantities, drums with inert atmosphere linings protect against both hydrolysis and contamination from plastics, which sometimes leach softeners into less robust containers.
Preparation Method
The method for preparing Tetrabutylphosphonium trifluoroacetate has evolved alongside the rest of ionic liquid science—what used to be a long, multi-step organic synthesis now gets done in days at a pilot scale. Most labs start with tetrabutylphosphonium bromide, reacting it with sodium trifluoroacetate in a controlled, biphasic system. Extraction relies on washing with deionized water, removing unreacted halide and related salts. Experience teaches that solvent choice for the phase transfer step shapes the yield—acetonitrile outshines methanol, especially as reactions scale up. The residual sodium salt washes out in multiple water rinses, while rotary evaporation strips away most solvent, leaving the dense ionic product ready for use or vacuum drying. Modern flow reactors have cut down the time needed, swapping batch for continuous production in larger plants.
Chemical Reactions & Modifications
This salt’s main claim to fame comes from its chemical stability, especially in acid/base regimes. In my own experience, very little seems to shift its structure. Adding strong nucleophiles or subjecting it to mild oxidation doesn’t often crack the phosphonium center, nor does it destabilize the trifluoroacetate. This ruggedness appeals to chemists working with sensitive catalysts, who benefit from a solvent that stays out of the way. Chemists sometimes tailor the phosphonium group—swapping alkyl chains or exploring mixed phosphonium-ammonium hybrids—but these tweaks rarely come close to displacing the tetrabutylphosphonium version. In rare cases, swapping the trifluoroacetate anion for bulkier or more electron-withdrawing carboxylates nudges the compound toward task-specific roles, particularly in biochemistry.
Synonyms & Product Names
The world of chemical trade names gets confusing fast. On MSDS forms and supply catalogs, this compound might show up as Tetrabutylphosphonium trifluoroacetate, TBP-TFA, or even the dense IUPAC moniker, Tributylbutylphosphonium trifluoroacetate. Some catalogs use the abbreviation [P4444][TFA]. Experienced buyers keep a wary eye for synonyms, as mismatched nomenclature sometimes masks subtle formula differences. For researchers just entering the field, cross-referencing with the CAS number cuts confusion—otherwise, the buy might go wrong.
Safety & Operational Standards
Like many ionic liquids, Tetrabutylphosphonium trifluoroacetate ranks low for acute toxicity, but that fact shouldn’t trigger complacency. The compound’s low volatility limits inhalation risks, yet gloves and eye protection matter, since slow leaching can disrupt skin over long exposures. Fume hoods help when heating or carrying out reactions under pressurized conditions. My time handling such salts taught the value of record-keeping—spilled product becomes slippery, and cherry-picking solvents for cleanup matters. Environmental guidelines push users toward managed waste streams; this isn’t a compound to send down the drain, as the fluorine content can build up in aquatic environments. Emergency protocols for fire pay special attention to decomposition fumes—phosphorus oxides and fluorinated gases form under sustained heat.
Application Area
Most chemical firms know Tetrabutylphosphonium trifluoroacetate not as a research toy, but as a core solvent and process additive. My own projects leveraged it in catalysis, especially for hydrogenation and cross-coupling reactions where traditional solvents stall out. Its non-coordinating anion rarely poisons metal centers, unlike more donor-rich buffers. Specialists in biomass conversion also experiment with this compound, seeing mild lignin solubility when other ionic liquids falter. Battery research leans into its electrochemical window, which edges out competitors for select electrode materials that corrode more easily. Some biotech labs use it during protein crystallization, counting on the gentle solvation for improved yields. Its anti-microbial properties spark interest among materials scientists; coatings and films get extended shelf life just by swapping in Tetrabutylphosphonium trifluoroacetate as a minor component. Not everything works—the salt won’t replace water-based solvents for most pharmaceutical runs—but its specialty uses keep niche markets alive.
Research & Development
Research dollars chase the next big application, and this compound often rides behind more famous ionic liquids. Still, publications over the past decade point toward growing interest in sustainability—low volatility and reuse win it points over chlorinated hydrocarbons. Industrial labs now routinely scan ionic liquids for CO₂ capture and electrochemical storage systems, bringing Tetrabutylphosphonium trifluoroacetate into the limelight. Some teams focus on tweaking the salt with functionalized moieties to address viscosity or tune conductivity without giving up on chemical stability. In university settings, professors push undergraduates to deploy the salt as a demonstration tool, since the low hazard profile keeps hands-on labs safer. Real innovation emerges from teams who embrace “salt-in-salt” systems, where one ionic liquid dissolves selectively in another, revealing physical behaviors far from standard textbook models.
Toxicity Research
Toxicity testing on Tetrabutylphosphonium trifluoroacetate still runs behind the more commercialized ionic liquids. What’s clear is that the salt’s acute oral and dermal toxicity scores stack up better than most classical solvents, though chronic exposure studies lag behind. Biodegradability remains a challenge — the trifluoroacetate anion tends to persist, since its carbon-fluorine bonds resist breakdown by microbes. In my own academic circles, we swapped to greener alternatives in cases where aquatic toxicity raised concerns, relying on closed-system recycling whenever possible. Manufacturers share this caution; they rank the compound as “potentially hazardous to aquatic life” in technical sheets and ship with clear disposal protocols. Some animal studies report mild liver impacts at large doses, fueling interest in alternate bench top solvents once quantities exceed research scale.
Future Prospects
Looking at coming years, Tetrabutylphosphonium trifluoroacetate sits at a crossroads. Regulatory pressure around perfluorinated compounds grows, yet functional demand in green chemistry won’t vanish quickly. What’s needed are head-to-head studies against newer “bio-based” ionic liquids, giving manufacturers and researchers confidence to either double down or pivot away. Scale-up remains tricky—the salt’s high viscosity taxes pumps, while waste handling costs give pause to would-be adopters beyond academia. Emerging applications in advanced batteries, carbon capture, and even microfluidic devices hint at growing markets. The big hope is that innovation in ionic liquid reusability, paired with honest analysis of risk, can unlock new frontiers. Anyone betting solely on “one size fits all” chemistry from this compound risks missing the point; nuanced, data-driven approaches will shape how Tetrabutylphosphonium trifluoroacetate moves from exotic lab staple to industrial mainstay.
A Closer Look at Uses in Chemistry and Industry
Tetrabutylphosphonium trifluoroacetate sounds complicated, but its job is pretty straightforward in the world of chemistry. Labs and companies across the world rely on it as an ionic liquid. People want to know why this catches so much attention. Its unique makeup lets it handle some roles regular solvents struggle with, especially when heat or chemical stability matters.
Key Role in Green Chemistry
My time in research labs showed just how important “green” alternatives can be. Traditional organic solvents often end up in waste and cause headaches for safe handling. Turning to ionic liquids—like this one—reduces volatile organic compounds you need to deal with. It’s also known for thermal stability and a surprisingly low vapor pressure, so folks near the bench don’t have to worry as much about fumes.
People use tetrabutylphosphonium trifluoroacetate in catalysis, extraction, and separation processes. For instance, when chemists want to react molecules in water but the usual solvents can’t handle it, this compound steps up. It dissolves a range of materials, including certain polymers and even cellulose, which is notoriously tough to break down. That opens doors for producing bioplastics or recycling plant-based waste in new ways.
Boosting Performance in Electrochemistry
Working with batteries and fuel cells brings its own set of challenges. I’ve seen researchers turn to this phosphonium salt to help electrolytes carry charge better or last longer. Good ionic conductivity and chemical resilience help batteries stand up to high temperatures. Friends in the field talk about mixing it into electrolytes to make charging smoother and reduce wear over time. On top of that, the trifluoroacetate part keeps the solution stable while it shuttles ions back and forth.
Why Labs and Manufacturers Pay Attention
Beyond the green angle, safety comes into play. Traditional solvents often catch fire or evaporate fast. I remember teachers worrying about routine spills or inhaling fumes from simpler chemicals. Tetrabutylphosphonium trifluoroacetate doesn’t evaporate much, and it stays stable under some tough experimental conditions. This means labs spend less time and money on specialized ventilation.
Cost and availability pop up in almost every discussion. It’s not as cheap as your everyday solvents, so routine use in massive plants might not make sense yet. Still, the price of safer and cleaner options keeps dropping as folks find better ways to manufacture them.
Paving the Way for Cleaner Chemistry
As demand for eco-friendly solutions grows, research continues on safer, less toxic ionic liquids. Scientists keep a close eye on toxicity and environmental impact before deploying new substances widely. Every team I’ve worked with runs their own toxicity checks, knowing regulations vary by country or region. This careful approach ensures safer workplaces and products down the line.
Adoption of tetrabutylphosphonium trifluoroacetate shows where chemical innovation heads next—toward balancing performance, sustainability, and worker safety. With more research and market interest, this compound stands as a reminder that better chemistry usually starts with listening to those using it every day and measuring up to real-world needs.
Cracking the Formula
Tetrabutylphosphonium trifluoroacetate shows up with a chemical formula of C16H36PF3O2. To make sense of that, a person might look at its building blocks. The tetrabutylphosphonium part means you have a phosphonium ion where four butyl groups attach to phosphorus. Each butyl group carries the formula C4H9. Stack up those four chains and you land at C16H36P for the cation piece. The other half, trifluoroacetate, comes in as CF3COO–, so three fluorines, two oxygens, and two carbons total. Put them together, you get the complete formula that often finds use across chemistry circles.
Why the Structure Gets Attention
Tetrabutylphosphonium trifluoroacetate is more than just numbers and letters. Its role as an ionic liquid gives it real-world impact. A compound like this stands out for research into green solvents, electrochemistry, and catalysis. In my experience working in academic labs, the right ionic liquid saves hours of frustrating trial-and-error. People look for stability, low volatility, and the ability to dissolve stubborn organic or inorganic compounds. This compound checks many of these boxes.
Researchers gravitate toward these salts because they mix well with a range of substances. In my own experiments running extractions, these types of phosphonium-based salts outperformed many classic solvents. They can dissolve metals, organic dyes, and difficult polymers in ways water or alcohol can’t manage. With regulations squeezing out volatile or toxic chemicals, a formula like C16H36PF3O2 earns its place on the bench.
Real-World Challenges
It’s easy for chemistry to stop at lists of compounds—labs keep things sterile, measured, far from daily life. Still, behind every new solvent or reagent looms a big question: Can it scale safely? Tetrabutylphosphonium trifluoroacetate works well in the lab vessel, but questions about toxicity, biodegradability, cost, and production footprint always hang overhead.
Looking at the phosphorus atom in this compound, for instance, it comes from a finite resource. Phosphorus mining places stress on the environment, and regulations over emissions and byproducts pile up year after year. Trifluoroacetate’s fluorine atoms offer both stability and environmental concern—the strong carbon–fluorine bond means slow breakdown after disposal, so waste handling and recycling need clear plans.
Paths Toward Safer Chemistry
Change can begin with better oversight in sourcing raw chemicals and safe disposal practices at every research or production step. Open data on environmental impact helps researchers pick greener candidates early in a project, saving headaches further down the road. Substituting renewable starting materials wherever possible keeps supply chains more secure. Institutions investing in recovery and recycling technology for ionic liquids could also lower long-term costs.
Some universities and private labs run pilot programs where they collect spent ionic liquids for regeneration. My own lab tested small-scale purification steps for solvents like this, aiming to reuse them several cycles before disposal. Even a simple database tracking solvent use can flag areas for tighter controls or smarter substitutes.
Moving Forward
People who work with chemicals like tetrabutylphosphonium trifluoroacetate know the value of getting the formula right, but a compound’s story runs longer than its appearance on the catalog page. By thinking not just about lab applications, but also about what happens before and after its useful life, the community can keep innovation and responsibility moving together.
Workplace Safety and Why it Matters
Anyone handling Tetrabutylphosphonium Trifluoroacetate knows preparation starts before opening the bottle. This chemical, often used in catalysis and advanced synthesis, carries risks that go beyond classroom theory. Safety is real, especially with liquids that promise both scientific breakthroughs and urgent problems if mismanaged.
Temperature and Light Matter More Than Labels
For many chemicals, a label might suggest room storage. That approach doesn’t cut it here. Tetrabutylphosphonium Trifluoroacetate asks for cool spots, far from sunlight and heat. I’ve seen labs treat the temperature range as a side detail, only to discover sticky residue inside cap threads and ghostly smells that trigger headaches. This isn’t just about protecting product shelf life—it’s protecting anyone who opens the container next.
Leaving this compound anywhere near heat sources spells trouble. High temps degrade ionic liquids unexpectedly, especially something as chemically active as this phosphonium salt. Steer clear of hotplates, sunny benches, or even shelves above radiators. Grab a chemical fridge set at 2–8°C. It keeps things stable, and you dodge a lot of unnecessary surprises. If you’re working in a shared academic or industrial space, log your container’s location—people forget, but chemistry rarely forgives.
Keep Moisture Far Away
Moisture changes everything with phosphonium salts. Store Tetrabutylphosphonium Trifluoroacetate in tightly sealed glass or HDPE bottles, always with a fresh desiccant pack nearby. I’ve seen colleagues get careless, leaving lids loosely screwed or skipping desiccant because “it’s just for a few hours.” Later, those same samples clump or show mysterious color changes. Water interacts fast at the molecular level, and that means your next reaction might fail or, worse, go somewhere dangerous. Don’t let a minor slip in storage sabotage months of research or production planning.
Ventilation Cuts Risk
Storage doesn’t end at the jar. Small leaks or accidental drops can send volatile compounds into the air, raising real hazards. Keep these chemicals in well-ventilated storage cabinets. Fume hoods or chemical storage rooms with mechanical exhaust guard against accidental exposure. Here’s the catch: relying on generic cabinets isn’t enough. Phosphonium salts like this can emit fumes—sometimes faint, sometimes pungent. People with sensitive lungs should take this seriously, and I’ve learned it pays to check vent fans and filters often. Maintenance logs matter as much as any lab protocol.
Label With Real Detail, Not Blank Jargon
Big red letters—write the chemical name, date received, and your contact info. Include hazard codes. A single unreadable label can spiral into confusion, especially during audits or emergency drills. From experience, legible and unambiguous labeling closes safety gaps faster than the best-written training materials. If you’re in charge of hazardous chemicals, spend time every week checking labels. It’s dead simple but often skipped.
Plan for Spill Response
Even with good storage, mistakes happen. Have spill kits, absorbents, and gloves right next to the storage spot. Don’t bury them under paperwork or random boxes. I’ve seen too many storage rooms where safety gear plays hide-and-seek. Reacting in seconds beats scrambling for supplies. Routine drills help too, reminding everyone what to do if things go wrong. This isn’t wasted time—it’s how you keep small mistakes from turning into emergencies.
Why People in Labs Pause Over Certain Chemicals
I’ve spent time working in research labs, and I know the first thing anyone does before opening a new bottle is check the safety sheet. Tetrabutylphosphonium trifluoroacetate might not shout trouble the way mercury or cyanide does, but it deserves a close look, especially for anyone who handles chemicals daily.
What Makes It Stand Out
This compound is not something you find under a kitchen sink. It’s a salt formed from tetrabutylphosphonium and trifluoroacetate ions. That structure means it belongs to the family of ionic liquids, used for some useful chemical reactions—particularly for “green chemistry.” Some labs have turned to these liquids to cut down on more notorious hazards like volatile organic solvents. The promise sounds good, but there’s another side.
Hazards That Shouldn’t Get Glossed Over
People sometimes assume a chemical is fine because it doesn’t catch fire easily or hit your nose with sharp fumes. This one doesn’t smell or spill like a solvent, but its components have other tricks. Trifluoroacetate ions raise concerns because some related chemicals linger in the environment and harm aquatic life. Trifluoroacetate itself is a breakdown product of some refrigerants and pesticides, and scientists still dig through research to understand long-term risks in water and soil. Persistent pollutants can travel far from the original source—nothing stays just in the beaker.
For the tetrabutylphosphonium side, research is less thorough. Worries come up about phosphonium compounds causing skin and eye irritation. Respiratory irritation can show up if powders or vapors escape. One spill of some ionic liquids on skin won’t cause trouble for everyone, but with enough contact, the story can shift. We don’t have as much long-term data on accumulation in people, either, so it’s smart not to relax your guard.
Fact-Based Risk, Not Hype
The global shift to new solvents always brings up the same situation: some companies tout lower toxicity with “green” labels, but research lags behind. I’ve seen teams get excited about alternative catalysts, then run into disposal issues as local authorities learn more about residues. Tetrabutylphosphonium trifluoroacetate doesn’t explode or stain skin like some older pool chemicals, but it isn’t as gentle as water. Treating any new salt as perfectly safe, just because it’s newer or less studied, reflects hope instead of evidence.
Better Approaches for Safer Workplaces
Lab folk need real guidance—not blanket assurances or panic. That starts with personal protective equipment for anyone handling these salts: gloves, goggles, lab coats, and proper ventilation. Anyone storing or using this salt should label it clearly and keep it away from acids or bases that could release hazardous gases. Spills need proper cleanup kits, and all waste needs containment for chemical collection instead of dumping it down the drain.
The last piece comes back to education. Students, techs, and even professors need up-to-date training on what they use. Old habits around “universal precautions” help, but reading the latest research or the safety data sheet keeps surprises in check. Until there's more research on biological impact—especially for aquatic systems—it makes sense to act as if these chemicals can bite.
Finding the Middle Ground
For anyone seeking a miracle chemical with no hazards, that search always turns out disappointing. With tetrabutylphosphonium trifluoroacetate, respect comes from clear information and honest discussion. Better rules, smarter storage, and routine care go a long way in keeping labs running and people safe.
Reliable Purity: What Buyers Look For
Anyone working with Tetrabutylphosphonium Trifluoroacetate knows that cleanliness in chemistry isn’t just about lab coats and clean benches; it’s about the specs on the label. Purity levels often reach 97% and higher. That figure isn’t just a marketing pitch—it separates a good reaction from a dud and keeps results from drifting into doubt. Low-level contaminants in something like this ionic liquid can change physical properties, gum up recyclability, and in extreme cases, throw a process off course. Companies invest heavily into quality assurance because, in areas from pharmaceuticals to advanced material science, trace residues can haunt downstream steps.
It might surprise some to find that most producers, especially those in Europe or the United States, hold their lots to fairly tight analytical standards. NMR and elemental analysis aren’t just checkboxes—they’re daily routine. Besides satisfying researchers, this careful approach meets the benchmarks set by regulatory bodies in industries such as electronics or biotechnology. Equipment doesn’t lie: a purity of 97%, 98%, or even 99% gets displayed on certificates accompanying shipments. These numbers tell a story about batch consistency—the sort that labs rely on for reproducibility.
Packing It Right: Various Sizes for Different Goals
Nobody orders chemical samples by the gallon when a few grams will do, just as full-scale production plants don’t mess around with vials. Tetrabutylphosphonium Trifluoroacetate comes in a range of packaging. Researchers usually go for 5-g or 10-g glass bottles, which fit perfectly alongside pipettes and microbalances. Specialty chemical distributors make it easy to source these sizes for academic work, exploratory synthesis, or analytic method development. Often, suppliers provide these aliquots in sealed amber or clear glass, which minimizes the risk of light degradation and keeps moisture out.
On the other end, process engineers and commercial partners often need kilograms instead of grams. Manufacturers offer bulk packaging—500-g bottles, 1-kg containers, and even up to 25-kg drums for those scaling up to pilot or industrial operations. Not every supplier handles large volumes, but companies specializing in ionic liquids usually have robust logistics for these needs. Plastic or stainless-steel drums get used for bulk, typically with secure liners to guard against leaks or contamination during transit.
Transport rules play a part here. Tetrabutylphosphonium Trifluoroacetate is not your run-of-the-mill solvent, so labeling follows hazard communication standards, and documents travel with every box or drum. Supply chains learned hard lessons from lost or damaged chemicals—the current approach means orders arrive with documentation that includes batch number, date, purity analysis, and storage advice.
What This Means for Users
Purity and packaging seem like simple features, but they influence how efficiently chemists, engineers, and manufacturers can put Tetrabutylphosphonium Trifluoroacetate to work. High purity doesn’t just make for better experiments; it saves time on troubleshooting and cleanup. Well-sized, secure packaging slashes the chance of waste and hazard. For people invested in risk management or lean production, these details keep costs down in the long run.
The industry moves forward by making these choices—secure supply lines, clear communication, and up-front purity data. If you’re handling this ionic liquid, expect to specify exactly what purity works best, right down to the decimal point, and select from bottle to drum depending on the job. Good chemistry builds on these details, every time.