Tetraethylammonium Trifluoroacetate: A Commentary
Finding Roots in the Evolution of Organic Salts
Tetraethylammonium trifluoroacetate didn’t just appear out of thin air—a string of discoveries in organofluorine chemistry, dating back to the middle of the last century, paved the way. Chemists in the 1950s grew fascinated by quaternary ammonium salts for their powerful use in phase-transfer catalysis. Around the same time, their interest in perfluorinated acids shot up, mostly because fluorine pushes molecules into new electronic territory. Ethyl groups, built onto a nitrogen core, hooked up with the strongly electron-withdrawing trifluoroacetate anion, tell a story of how researchers pushed boundaries to achieve better control in organic synthesis and analytical science. Thinking back to university days spent in labs, one truth kept surfacing: each seemingly niche compound on the shelf opens more possibilities for precision in chemistry.
Product Overview: Purpose Meets Practicality
Tetraethylammonium trifluoroacetate comes as a white, crystalline solid. It dissolves well in water and polar organic solvents. Its role shines in situations calling for a reliable electrolyte or phase transfer agent. In instrument calibration and analytical assays, accuracy always depends on the stability and predictability of the ingredient. Businesses making specialty chemicals keep it around for precise ion-pair chromatography, where the goal isn't just separation, but confidence that every analysis tells the real story. Chemical supply companies label it with technical grades, usually keeping impurities such as chloride or moisture below one percent, and always list typical batch-by-batch variations to meet the demands of certification in regulated settings.
Physical & Chemical Properties: What Sets It Apart
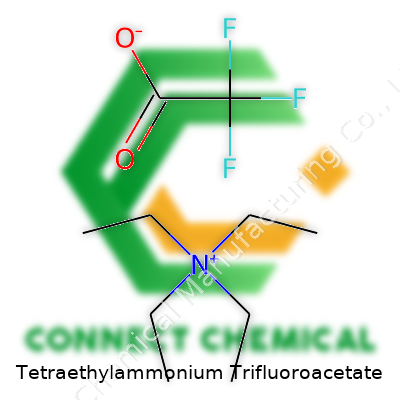
With its formula (C8H20N)+(CF3COO)-, tetraethylammonium trifluoroacetate boasts a molecular weight around 277 g/mol. Flammability concerns stay minimal, but good ventilation makes sense, since inhalation of trifluoroacetic acid’s breakdown products can irritate the lungs. The salt melts at moderate temperatures—usually above 100°C. Its structure balances hydrophobic tetraethylammonium and the highly polar trifluoroacetate. That unique combination finds use where you need both ionic strength and good solubility in organic media. From experience, storing it dry and in airtight containers helps preserve its shelf-life, keeping both caking and unwanted hydrolysis at bay.
Technical Specifications and Labeling: Getting Granular
Labels stand out as tools to keep labs and warehouses safe and organized—not just paperwork. Each bottle usually lists the precise chemical composition, purity percentage, possible contaminants like halide traces, and recommended storage guidelines. Chemical Abstract Service (CAS) numbers appear alongside warning icons for skin and eye safety. Certain suppliers now print QR codes on the bottles so labs can pull up safety data sheets, batch analysis, and expiration dates from their phones. As a researcher juggling chemicals, the clearer and more complete the label, the fewer mistakes occur under pressure.
Preparation Method: Blending Precision with Know-How
Chemists prepare tetraethylammonium trifluoroacetate through a metathesis reaction. Stirring an aqueous solution of tetraethylammonium bromide with silver trifluoroacetate causes a simple swap: insoluble silver bromide drops out, leaving behind the desired product in solution. Filtration and careful evaporation deliver the pure salt. Scaling up means handling both silver-containing waste and keeping the trifluoroacetate dry, as moisture in the work-up can lead to hydrolysis–spoiling entire batches. Doing it right the first time, rather than racing through and getting sloppy, saves more than chemicals—it keeps personal and team safety uncompromised.
Chemical Reactions and Modifications: Versatility on Display
In organic chemistry, tetraethylammonium trifluoroacetate brings value to reactions needing selective ion pairing. It can tune the solubility of ionic intermediates and sometimes features as a partner in nucleophilic substitutions, especially in fluorine-rich environments where the trifluoroacetate will either stabilize or shuttle ions. Modifying its basic structure—swapping out the ethyl groups for other alkyl chains, for example—opens doors to salts with different melting points and solvent compatibilities. It’s a favorite for experimentalists developing new electrochemical protocols or chromatographic separations, because a small tweak in structure can make a measurable difference in performance.
Synonyms and Product Names: Speaking the Same Language
Over the years, folks working across industries and borders have picked different names for this compound. In catalogs, you’ll see “TEA trifluoroacetate,” “tetraethylammonium trifluoroacetate,” or “TEA-TFA.” Sometimes, abbreviations wander in scientific papers, leading to confusion if researchers don’t double check. Making sense of synonyms keeps miscommunications to a minimum, a lesson drilled into any lab manager who’s ordered the wrong variant at least once and learned the hard way.
Safety and Operational Standards: Prioritizing People and Process
Like most fluorinated chemicals, respect is a must when working with tetraethylammonium trifluoroacetate. Wearing gloves, goggles, and using a fume hood prevents accidental contact or inhalation. The Material Safety Data Sheet flags it as an irritant with acute effects on mucous tissue. For waste disposal, neutralizing solutions before sending them through regulated hazardous waste channels follows both law and best practice. Staying alert to these standards isn’t just about ticking off a safety checklist—it reflects an attitude in the lab where attention to detail protects everyone in the room. Recurring safety training and signage help reinforce those habits, especially when new staff join and might not recognize hazards on sight.
Application Area: Going Beyond the Obvious
Electrochemists turn to tetraethylammonium trifluoroacetate for its clean ion mobility and solvent versatility. Analytical labs use it in capillary electrophoresis buffers and for ion-pairing in high-performance liquid chromatography, especially in pharmaceutical assays for separating charged drugs. Synthetic organic chemists favor it in fluorination reactions or as a non-nucleophilic counterion that won’t get chewed up in side reactions. Peptide chemists have tested it in purification stages, where other salts can leave sticky residues or disrupt mass spectrometry analysis. Having witnessed its flexibility first-hand, it has clear staying power in labs chasing both routine checks and pioneering new chemical transformations.
Research & Development: The Drive for Better Tools
Research investment keeps growing in the field of ionic liquids and next-generation electrolytes, with tetraethylammonium trifluoroacetate playing a part as a model compound. Developers study it as a benchmark for conductivity and electrochemical windows. Its clean reactivity profile helps in building datasets needed for AI-based prediction of salt behaviors—a big deal in pharmaceutical and energy storage R&D. Real conversations among researchers highlight a shared longing for more biocompatible or less persistent alternatives. Academic and commercial teams collaborate across continents, racing to find the next leap: maybe by switching out the ammonium core, or using greener synthesis routes.
Toxicity Research: Protecting More Than Just Chemists
Published studies show that quaternary ammonium salts need careful handling. Some older reports paint them as cytotoxic in high doses, impacting cell membranes and nervous system function. Trifluoroacetate brings its own baggage, since it’s persistent in the environment and can build up in water sources. New toxicity tests—not just on rodents, but using human cell lines—keep refining our picture of how safe this salt really is for long-term use. Data transparency means that suppliers update safety datasheets as soon as they spot new risks. In practice, that drives a culture of minimizing unnecessary exposure and waste, which isn’t just legally required—it's the right thing to do.
Future Prospects: Pushing Toward Sustainability and Performance
Tetraethylammonium trifluoroacetate stands at the crossroads of legacy chemistry and evolving needs. Researchers want its reliable physical properties but push for greener synthesis methods and lower toxicity profiles. Companies look at alternative anions, less persistent in water, and try bio-based feedstocks for the cation. The next five years promise more accurate predictive modeling, better regulatory data, and new recycling solutions for used salts. Watching the field change from the inside, it’s clear the journey isn’t just scientific—it’s about keeping pace with tougher environmental rules and customers demanding transparency.
Everyday Chemistry at Work
Tetraethylammonium trifluoroacetate doesn’t come up in most conversations, but anyone spending time in a research lab or a chemical plant will recognize its value. It brings together two pieces that seem unrelated: tetraethylammonium, a positively charged chunk with four ethyl groups hanging off a nitrogen atom, and trifluoroacetate, a small but punchy anion packed with three fluorine atoms next to a carboxyl group. You get the formula: (C2H5)4N+ CF3COO–, or written out as C8H20N·C2F3O2. This seems like a string of letters and numbers, but each piece signals a specific job.
Molecules with Real Jobs
Both parts earn their keep in the world of materials science and biochemistry. In my work in the lab, tetraethylammonium pops up anytime researchers need a strong, stable cation for conducting electricity in unusual solutions, often when exploring properties of new batteries or cutting-edge solar cells. Battery fluid won't deliver if the cation falls apart or latches onto the wrong neighbor. Tetraethylammonium holds up—steady hands, no drama.
Trifluoroacetate brings its own flavor. Three tough fluorines keep the anion from playing the troublemaker, resisting unwanted reactions and keeping things clean. Regulating charge balance and keeping the real action (like electron transfer or catalysis) from getting sidetracked, trifluoroacetate steps back and lets the science happen. In protein studies, I've seen teams use it to adjust pH or as a counterion that won’t mess with measurements.
Trust Built on Experience and Safety
Chemicals like tetraethylammonium trifluoroacetate keep showing up in labs thanks to decades of tried-and-tested experience. The main challenge lies in handling—they demand respect. Tetraethylammonium pieces, not exactly gentle, need careful labeling and storage, especially since improper handling, inhalation or skin contact can lead to health concerns. Vigilant policies and strong safety culture pay off. I've seen new techs learn the ropes using straightforward procedures, with a real focus on gloves, goggles, and smart storage. These lessons save accidents and keep newcomers out of harm’s way.
What Makes the Formula Matter Now?
With names as long as a shopping list, formulas can seem like technical clutter. But breaking down the formula tells researchers what the molecule brings to the table: dependability as an electrolyte, steadiness as a pH buffer, or performance in a protein purification setup. The shortfall comes if teams treat every molecule as just another interchangeable part. Years of practical experience—mixing, measuring, troubleshooting—build an understanding of how even a small change, like switching out trifluoroacetate with another counterion, ripples through a whole experiment. One swap changes solubility, pH, and often the final result.
Smarter Solutions and Better Outcomes
Many of the best solutions begin with respect for the building blocks. Relying on chemicals with clear records and solid formulas lets scientists focus on discovery rather than damage control. Tight documentation and good supplier relationships help with reliability—labs pick suppliers who meet regulatory and safety standards, who back up their products with data, and who stay responsive if problems crop up. Inventory systems with real-time tracking help teams stay ahead of shortages and avoid expired stock.
The more we share knowledge about substances like tetraethylammonium trifluoroacetate, the more we help the next crew work safely and effectively, with fewer surprises and stronger results.
Chemistry Labs and the Real Need for Reliable Agents
Every chemist craves reliability. In my years sharing lab space, few compounds spark tech talk like tetraethylammonium trifluoroacetate. It doesn’t make headlines, but people in synthesis, electrochemistry, and analytical circles throw the name around with real purpose. Why? This salt puts out a performance that matters to anyone dealing with ionic reactions and chancy solvents.
Shifting the Balance in Electrochemical Studies
I’ve lost count of experiments where cationic strength quietly wrecks hours of work. In electrodeposition or battery testing, a supporting electrolyte helps ions move without clogging results. The big tetraethylammonium cation matches that need, shuffling between electrodes without sticking or reacting with other pieces in the setup. Pull out the wrong salt, and background noise drowns out your signals. With the extra electron-withdrawing fluoroacetate partner, solutions stay stable and sharp — the update comes through in real time on any half-decent voltammetry screen.
Organic Synthesis: Not Just Boring Salts
Molecular tweezers work best with steady hands. Protecting groups fall off, catalyst cycles stall out — half the time, weak ionic control ruins what should be a one-step clean conversion. Tetraethylammonium trifluoroacetate fills a role as a phase-transfer agent, shuttling reactants across phases in biphasic systems while refusing to participate in the reaction. Where other quaternary salts add water, clouding up byproducts, this one’s high-salt, low-water nature turns what could become a chemistry mess into a repeatable tool for carbon–carbon coupling or halide substitution. The trick is finding solvents and partners it can handle, but for the challenging ones, it proves itself pretty quickly on the bench.
Protein and Nucleic Acid Work: More Than Buffers
Years working with mass spectrometry taught me to respect little details. Protein samples desalt poorly; peptide analysis drags along all sorts of noise from common buffers. Tetraethylammonium salts, especially as trifluoroacetate, pop up in mobile phases for LC-MS and ion-exchange chromatography. Because of the large tetraethylammonium ions, the compound prevents sample clumping and helps keep complex peptides separated. Trifluoroacetate’s strong acidity also makes sure that fragments stay ionized enough for detectors to catch them — something I’ve seen play out when older acetate salts just didn’t cut it.
Downsides and Responsible Chemistry
No point skipping the less shiny side of things. Organofluorine waste builds up, and many labs still toss out old solutions like nothing. Chronic exposure risks can’t just be handwaved if you share air with more than a fume hood. I learned the hard way to respect gloves and spill protocols, because salts like these don’t wear warning labels for fun. So the broader chemistry community faces a puzzle: How do we hang on to the advantages of designer salts without handing off tomorrow’s headaches to someone else? Safer disposal, more local recycling, and looking again at even “harmless” additives might make the difference between smart advancement and future regret.
Looking Forward: Research with a Purpose
Having a tool like tetraethylammonium trifluoroacetate changes the rhythm of research. In synthesis, analytics, and energy labs, small differences in salt choice stack up to better results and fewer ruined batches. But with every new specialty chemical, responsibility grows. Chemists and lab managers who value transparency and long-term safety — not just performance today — will chart the path for smarter uses tomorrow.
The Real Risks in the Lab
Working with chemicals like Tetraethylammonium Trifluoroacetate takes more than just following a procedure out of a manual. This compound crops up in research projects tied to organic synthesis and sometimes in segmental biomedical research. My own years spent in university labs gave me a front-row seat to how easy it gets to cut corners or distract yourself, even with substances that could bring on harm fast. No matter how familiar you get, this isn’t table salt. This chemical demands respect from anyone handling it.
Why The Right Gear Matters
Diving straight in, gloves and goggles don’t exist for show. The trifluoroacetate part of the molecule can irritate eyes and skin, especially over longer exposures, and no one wants to spend the afternoon washing out their eyes. Entry-level gloves—like nitrile, which stands up to a wide range of solvents—form the first physical barrier. Lab coats and long pants stop accidental spills from soaking through. Splash-proof goggles don’t only help for sudden splashes; even brief vapor exposure close to your face adds up. In small spaces, these little lapses matter a lot.
Vents and Fresh Air Still Save the Day
Plenty of people skip the fume hood when they think something isn’t noxious at room temperature. That choice bites back. Tetraethylammonium Trifluoroacetate gives off vapors that linger and spread, aggravating lungs and mucous membranes. Active ventilation clears out vapors before they build up enough to threaten nose, throat, and even your sense of comfort in the lab. Purging the workspace with fresh air and keeping the hood sash down lower shrinks exposure risks drastically, and every chemist who cares about making it home healthy follows this step as habit.
No Room for Guesswork: Labeling and Storage
Piling up clear, specific labels on every bottle and flask isn’t just about neatness. During my grad school days, I watched a visitor splash the wrong chemical into an open beaker because the cap “looked familiar.” No one wants to scrub a bench all afternoon trying to fix a mix-up or, worse, run an experiment that exposes everyone. Storage in dry, cool cupboards keeps decomposition in check, and separating highly reactive chemicals guarantees stray vapors don’t meet up and spark new problems. Organizational skills—good labeling, tidy storage, regular inventory—turn into genuine safety tools.
Spill Response and Skin Contact
Simple soap and water aren’t always enough for a trifluoroacetate spill. Quick action turns a bad spill into a minor cleanup. Absorbent pads, specialized spill kits, and protocols for hazardous waste disposal remain the gold standard. For small skin exposures, immediate flushing under running water goes further than most people realize, but large contact means dialing the emergency shower and seeking medical help without delay. It pays to keep first aid stations stocked and visible, so hesitation never sets anyone back in the case of an emergency.
Proper Training—No Shortcuts
Training doesn’t stick if people don’t care about why each rule exists. Entry-level training that explains Tetraethylammonium Trifluoroacetate’s properties, routes of exposure, and what to do in an emergency lays the groundwork for a safe routine. Returning to regular, bite-sized refresher courses anchors good habits and keeps safety knowledge fresh instead of letting it fade into background noise. Real safety never just happens; it is built, one smart step at a time.
Understanding the Material You’re Working With
Anyone with lab experience knows some chemicals ask for special attention. Tetraethylammonium trifluoroacetate doesn’t jump out as a fireball hazard at first glance, but it’s no table salt. Its trifluoroacetate moiety hints at volatility and corrosiveness, while the tetraethylammonium cation brings flammability risks into play. Overlooking safety details never did any chemist any favors. At grad school, I saw someone stash a similar quaternary ammonium compound in a hot corner near a sunlit window. A month later, that bottle’s cap had cracked, and we had fumes stinging our eyes. That sort of oversight turns a boring routine into an emergency.
Temperature and Humidity Aren’t Just Numbers
Tetraethylammonium trifluoroacetate holds up best in a cool, dry, and stable spot. Laboratories set clear temperature limits for chemicals—usually 2°C to 8°C for sensitive salts. I learned early to double-check thermostats on fridge units, because a silent malfunction turns pricey reagents into useless slurries. Heat sparks reactions in trifluoroacetate, and humidity allows water to creep in, turning salts into sticky messes. If you store this salt near a sink or open glassware, chances jump for contamination. That can spoil months of synthetic work or make anyone sick through unplanned exposure.
Why Sealed Containers Can’t Be Skipped
Letting air reach tetraethylammonium trifluoroacetate opens the door for hydrolysis and corrosive vapor release. Good practice seals this type of compound in airtight glass or PTFE screw-cap bottles. My own bench experience taught that even a tiny crack along a lid caused odors to seep out and product to break down faster. Foil-lined seals or Parafilm can back up older closures, extending reagent lifespan. Sometimes, a mole of moisture can change a salt’s performance in a catalysis run—something you only catch after finding peaks missing in your NMR stack.
Labeling: A Step Worth Doing Right
Every time an unlabeled or faded bottle shows up, someone takes a risk. Labeling seems simple, but in a hectic lab, clear hand-written dates and hazard codes give future users a fighting chance. I watched a visiting scholar mis-identify a bottle and spoil a full day’s prep because the right warning got covered up with condensation. Tough labels designed for freezer and solvent handling take the beating, while basic marker ink fades with a few glove changes.
Access Control and Monitoring Make a Difference
I’ve worked in labs where a five-minute search for basic chemicals wasted everyone’s time. Designating a spot and logging each use, even in a scribbled notebook, keeps surprises down and accountability up. Some teams use RFID or barcode systems, but discipline matters more than gadgets. Checking stock and container condition weekly prevents accidental discoveries of degraded or spilled chemicals. Missed audits grow into full-on clean-up jobs, sometimes with professional support.
Reducing Waste and Exposure
Ordering smaller amounts and prepping single-use aliquots cut risk and conserve money. No one enjoys discarding expensive waste. I once watched a group split a single batch into several amber vials, each sealed under dry nitrogen. Losses dropped. Accidents fell too. That’s a habit worth repeating, especially with reagents that degrade or fizz with moisture.
Final Thoughts on Safe Habits
Safe storage grows from paying attention to basics. Separating incompatible chemicals, like strong bases or acids nearby, can avoid unwanted reactions if a spill happens. Protective gloves, fume hoods, and well-designed spill kits aren’t overkill. They’re the difference between a regular day and a story you'll want to forget. In my own work, leaning into habits that keep everyone healthy ended up saving time and letting the science run smoother.
The Real Story on Solubility
Tetraethylammonium trifluoroacetate steps into the lab as a salt that makes chemists pay attention, mainly because of its unique structure. Every chemist knows not every salt plays nicely with every solvent, and this one proves the rule. Toss this chemical into a beaker of water and you’re not left waiting long. Water does the job right, dissolving it efficiently. This tracks with what we expect—the tetraethylammonium ion brings enough organic bulk to keep things interesting, but the trifluoroacetate part carries decent hydrophilic flair thanks to those fluorines. In my own time at the bench, salts with this sort of set-up almost always vanished into water swiftly, and Tetraethylammonium trifluoroacetate is no exception, with reports noting strong solubility upwards of several grams per milliliter.
Not the Same in Every Solvent
Pick up some polar aprotic solvents — acetonitrile, dimethyl sulfoxide (DMSO), N,N-dimethylformamide (DMF) — and you’ll get a different story. Acetonitrile can usually handle tetraalkylammonium salts, and that’s the case here. DMF and DMSO also dissolve it well, fitting with their ability to break up salts. Using it in less polar organics like ether or hexane? Forget it. Here you run into a brick wall, the salt stays stubbornly solid, and the solution stays clear. Too little polarity.
Why This Matters to Researchers
This solubility profile matters in the real world. For anyone running electrochemical studies or prepping NMR samples, solvent choice isn’t just about convenience. The success of catalysis or signal clarity often comes down to how well your chemical goes into solution. I've seen experiments grind to a halt when a salt wouldn’t dissolve after mixing for an hour—nobody working with Tetraethylammonium trifluoroacetate in water or strong polar solvents needs to worry about that headache.
It's worth noting that temperature shifts can improve things. Warmer water or acetonitrile increases solubility further, which comes in handy during prep. Excessive heat sometimes risks decomposing sensitive salts, so moderating temperature helps preserve your chemical and your data.
Brief Science Behind the Numbers
The easy dissolution in water and DMSO comes down to ion-dipole interactions. Tetraethylammonium cations and trifluoroacetate anions split up under polar conditions, stabilized by plenty of tiny solvent hands grabbing on. In less polar solvents, those hands let go, and you’re left with clumping, undissolved salt. Data from the literature, including product datasheets and the Merck Index, back up this consistent pattern. Chemists can usually find solubility data for similar salts, and the numbers typically fall in the ballpark of tens of grams per 100 milliliters for water and strong polar organics. Hexane, toluene, and other low polarity choices never break a gram, usually not even a tenth.
Smart Solvent Choices and Common Pitfalls
Working with this salt, going for water, methanol, or DMSO skips most headaches. As much as I appreciate the adventure of tweaking solvent mixes, there’s rarely much to gain with tetraethylammonium trifluoroacetate outside the tried-and-true options. Watch for salt precipitation when switching solvents or concentrating solutions—this can wreck your reaction or precipitate out essential ions. Keeping a test tube on hand for quick solubility checks has saved me more than once from botched preparations.
For anyone heading to the bench, remember: a little research beats hours hunting down the “right” solvent. Checking a couple of references and leaning on chemistry’s long memory—plus a colleague’s advice—goes a long way toward making the most of this versatile salt.