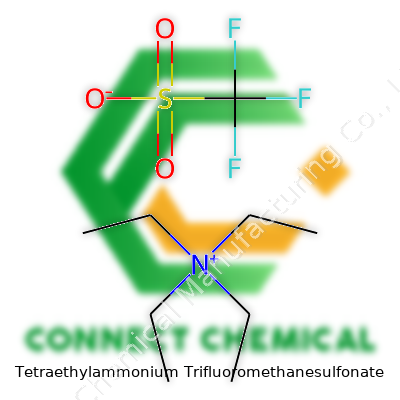
Tetraethylammonium Trifluoromethanesulfonate: A Commentary on Its Evolution, Role, and Future
Historical Development
Tetraethylammonium trifluoromethanesulfonate entered the scene as scientists hunted for better ways to drive ionic movement without bringing in the downsides of metal cations. Over the past decades, researchers have depended on this salt across many electrochemical experiments, finding it effective in applications from synthetic chemistry to the nuts and bolts of battery design. Its roots reach back to a time when chemists experimented with a long list of ammonium-derived salts as supporting electrolytes for conductivity in nonaqueous media. Study after study pointed out that tetraethylammonium stuffed with a triflate anion offered superior solubility and thermal stability, pushing it beyond alternatives like perchlorates and halides. Universities and laboratories built a toolkit around it, and supply chains followed.
Product Overview
Many in research and industry recognize tetraethylammonium trifluoromethanesulfonate by its clean, non-hygroscopic, and crystalline nature. Researchers handling this salt usually come across a white or slightly off-white powder, packed in airtight bottles or aluminum foil packets to avoid moisture pick-up. The importance of a dry, pure version can’t be overstated in synthetic chemistry. This compound doesn’t just fill a niche; it’s a staple for those who need a non-coordinating, stable counter-ion to power electrochemical reactions or craft advanced materials.
Physical & Chemical Properties
What stands out about tetraethylammonium trifluoromethanesulfonate is its respectable melting range, hovering around 135-138°C, with a robust decomposition temperature. It dissolves freely in polar organic solvents—acetonitrile, dimethylformamide, and methanol feature among the usual suspects. Its signature lies in the partnership between the bulky tetraethylammonium cation, which resists coordinating with transition metals, and the delocalized triflate anion, which brings non-nucleophilic behavior. Together, they drive high mobility and stability under lab and industrial conditions.
Technical Specifications & Labeling
A closer look at reagent bottles will reveal purity levels typically climbing above 98%. Most labels specify water content, halide traces, and heavy metal limits—these aren’t arbitrary figures, but strict demands from electrochemists who can’t risk contamination or unwanted side reactions. Handling instructions almost always flag the need to store the compound in tightly sealed containers, out of direct sunlight and damp air. I’ve seen inconsistent labeling practices across vendors, so cross-checking certificates of analysis with independent test data has become a best practice among careful labs.
Preparation Method
The classic preparation calls for reacting tetraethylammonium hydroxide with trifluoromethanesulfonic acid. This acid-base neutralization delivers tetraethylammonium trifluoromethanesulfonate in solution, typically followed by concentration and drying under vacuum. Purification by recrystallization from acetonitrile yields material clean enough for most research. Large industrial batches may rely on ion exchange or alternate anhydrous synthesis to prevent decomposition or side-product formation. The sheer routine nature of the process belies its importance—a less careful approach, especially with acid concentration, risks leftover water or decomposition that can sabotage sensitive research.
Chemical Reactions & Modifications
Tetraethylammonium trifluoromethanesulfonate walks a careful line: it’s robust against decomposition under mild conditions, favors ionic over covalent behavior, and stands up against a spectrum of electrophilic or nucleophilic reagents. I have seen it employed as an efficient electrolyte in synthesis of organometallic complexes, where traditional salts fail due to undesirable ion pairing. It can transfer the triflate group in some creative organic transformations, especially under strong acidic or basic regimes—though most chemists prefer to keep it as a spectator ion that stirs the pot without much fuss. Side reactions generally mean hydrolysis in the presence of water or decomposition at elevated temperatures.
Synonyms & Product Names
Depending on the supplier or research context, you’ll see this salt under a handful of names: tetraethylammonium triflate, TEATf, TEAOTf, or via its formal IUPAC title. In catalogs, it will often appear alongside variations in solvent or dilution format—though for demanding studies, pure dry powder is the only acceptable option. Mixing up trade names with structurally similar salts like tetraethylammonium perchlorate has caused headaches in more than one research group. Keeping an eye on batch numbers, source, and even minor changes in labeling prevents confusion and protects experiments.
Safety & Operational Standards
Lab safety posters underestimate the importance of proper handling, especially with dry powders like tetraethylammonium trifluoromethanesulfonate. Direct contact on skin or mucous membranes shouldn’t be ignored, since strong ammonium salts can irritate and accidental exposure through dust easily happens during weighing. Proper ventilation, gloves, safety glasses, and a habit of quick cleanup make a difference. Storage in dry, airtight containers sits among the first recommendations on most safety data sheets. Disposal protocols require collection in designated waste streams to prevent environmental release, reflecting growing scrutiny on laboratory micro-waste. Compliance with REACH and GHS labeling matters—critical in the eyes of regulators and anyone whose work could lead from the benchtop to industrial scale-up.
Application Area
Electrochemistry departments prize tetraethylammonium trifluoromethanesulfonate as a supporting electrolyte in nonaqueous solvents. It pops up in studies of anodic oxidations, redox catalysis, and battery prototypes. Synthetic chemists use it to enable reactions that call for truly non-coordinating ions, bypassing problems tied to more reactive cations. Among newer directions, its role as a dopant for organic electronic materials or a stabilizer in ionic liquid formulations opens doors for innovation in fields spanning from green energy to pharmaceuticals. At the interface of biology and chemistry, careful use has allowed probing of nerve channel blockers and enzyme mechanisms. The take-home point: if the experiment needs clean, stable, and efficient ionic movement, this salt wins attention.
Research & Development
The scientific literature brims with examples where tetraethylammonium trifluoromethanesulfonate gave traditional methods a shot in the arm. Recent papers spotlight its role in unlockable redox windows, supercapacitor prototypes, and new approaches to catalysis. My own reading of the field shows an uptick in patents exploiting its properties in electronics or as a mediating ion in advanced organic transformations. Industrial-scale developers continue to refine its production with greener methods—switching from volatile organic solvents to water or solvent-free grinding—for both health and environmental benefits. Multi-disciplinary teams now examine how this compound’s peculiar ionic profile can unlock next-generation batteries or flexible electronics.
Toxicity Research
Awareness of toxicity goes beyond the safety sheet. Limited animal studies hint at moderate acute toxicity if ingested or inhaled, with classic ammonium irritation signs—redness, burning, or local tissue disruption. Chronic exposure information stays sparse, urging a conservative approach common to all ammonium derivatives. Handling with respect, keeping dust from airborne dispersal, and scrupulous decontamination have prevented major lab incidents to date. Regulatory agencies, faced with fragmentation in toxicology data, continue to urge users to minimize release and monitor for occupational exposure, especially in industrial settings where handling scales up dramatically.
Future Prospects
Upcoming years look bright for tetraethylammonium trifluoromethanesulfonate. As labs pivot from traditional lithium and sodium salts toward less reactive, more stable ionic frameworks, research points to new applications in sustainable energy, organic electronics, and green synthesis. Supercapacitor and battery researchers already lean on its stability and low reactivity as they hunt for safe and long-lived storage devices. I’ve noticed a rising tide in innovation toward cleaner production routes, replacement of toxic counter-ions, and tight lifecycle analysis to answer calls for responsible chemistry. Educators and lab managers can expect demand to climb—and as that happens, emphasis will fall on rigorous quality control and sound stewardship at every step from synthesis to disposal.
Beyond Complicated Names: What Stands Behind This Salt
Some chemicals carry a name that makes anyone want to skip to the next paragraph. Tetraethylammonium trifluoromethanesulfonate falls right into that category. But push past the "tongue-twister" effect, and this substance lands squarely in the middle of real, practical science. I’ve spent enough time around labs to know that simple-sounding salts hide surprising jobs. This one gives researchers and industry folks a strong tool, not for anything most of us find in the grocery store, but for deeper work, where details matter.
Driven By the Charge: Electrolytes and Why They Matter
In the lab, chemists care about ions—tiny charged particles that help carry electricity and drive reactions. If you crack open a battery or look at most electrochemical experiments, you’ll find electrolytes at work. Tetraethylammonium trifluoromethanesulfonate falls into the family of “ionic salts” that don’t just dissolve in water. Drop it in solvents chemists favor, like acetonitrile, and it completely breaks apart into ions, making it perfect for research on conductivity and electrochemical cells.
This means any group studying how electricity flows across a liquid, or how materials hold a charge, usually stocks this chemical on their bench. I’ve run my share of electrochemical tests, and reliable salts make all the difference between crisp data and head-scratching noise. This one rarely lands in everyday batteries, but it tells researchers how better materials might perform or how molecules act under different electric fields.
Shaping Tomorrow’s Electronics—and Even Medicine
Most people never see this salt, but its fingerprints show up in unexpected places. Scientists working on organic electronics—think of flexible displays or new solar panels—turn to this compound because it creates conditions that mimic real-life charge movement. It acts as a sort of backstage hand in the world of molecular electronics, pushing the limits on how small and flexible devices can get.
More surprising to those outside research: this salt helps probe how blood vessels and nerves send signals. Tetraethylammonium as a compound helps block potassium channels in nerves—this gives neurobiology labs a tool to tease apart how nerves fire or how drugs affect the body. Pair that with the trifluoromethanesulfonate part for better water solubility, and you’ve got yourself a compound that fits right in with biological research.
Keeping It Safe and Smart
Diving into chemicals like this means more than turning a pipette. Each handling step—measuring, dissolving, storing—matters. I learned quickly that these salts stay far from kids, pets, or careless spills. Gloves, goggles, and good ventilation aren’t a formality—they protect from accidental exposure, and the triflate part brings some risks. Ignore basic care, and you invite headaches or worse. Whether preparing a solution for a conductivity test or working on a new polymer, lab safety never feels optional.
Future Steps Start Now
Better batteries and greener electronics demand better electrolytes. The research keeps moving. If cost, purity, or environmental impact raises problems, we need new salts or clever recycling programs. Universities and companies keep pushing, because each improvement adds up: lower power losses, safer devices, cleaner labs. Complex names aside, practical impact starts with the choices chemists make every week.
Understanding the Formula
Chemical names can look intimidating, but they tell a clear story if you break them down. Tetraethylammonium trifluoromethanesulfonate sounds like something best left to specialists, yet its formula makes sense piece by piece. You get one part tetraethylammonium, which is (C2H5)4N+. The other part is trifluoromethanesulfonate, known by many as triflate, with the formula CF3SO3-. Put them together as a salt, you end up with C8H20N.CF3SO3 or, written simply, C9H20F3NO3S.
Getting the Molecular Weight
Quick calculations on a scrap of paper or with any decent digital calculator make life easier for students or anyone heading into the lab. Here it breaks down: carbon (12.01) times nine, hydrogen (1.008) times twenty, fluorine (18.998) times three, nitrogen (14.01) times one, oxygen (16) times three, and sulfur (32.07) times one. Punch the numbers in and it all adds up to 297.32 g/mol.
Why This Salt Isn't Just a Curiosity
Once you get past memorizing formulas, you notice tetraethylammonium trifluoromethanesulfonate popping up in more than a few research papers. It acts as a neat and stable electrolyte in electrochemistry experiments, especially those pushing the boundaries of battery science. I remember working with this in organic synthesis labs because of its reliable solubility in polar solvents and its knack for not interfering with delicate reactions. Many grad students can recall pouring it from a bottle that cost a chunk more than its cousins because high purity really matters at that stage.
Anyone with a foot in pharmaceutical R&D, green energy, or materials engineering has a reason to know exactly which salt they're dropping into a reaction flask. This compound’s non-coordinating triflate anion helps avoid reaction hiccups that crop up when certain ions bind too tightly. A lot of colleagues keep an extra stash around for NMR studies—using it as a reference or as an agent to boost dissolution.
Why Accurate Data Makes a Difference
Getting the formula right matters. Slipups with chemicals can mean wasted hours, trashed experiments, or even safety mishaps. Attention to molecular weight isn’t just academic. In the past, labs that misjudged measurements saw entire projects stall while repeat orders, recalculated solutions, or re-run HPLC results sorted the mess out. Reliable chemical suppliers know their clients look for accurate CAS numbers, formulas, and molecular weights for each compound—otherwise, the trust just isn’t there.
Most folks aren't running personal electrochemical cells or doing ionic conductivity tests at home, but for the people in those roles, knowing compounds like this one inside out lets them push the next layer of innovation. For undergrads starting out, it’s good practice: know your chemical, why you’re using it, where its numbers come from, and how those details affect every single outcome.
Best Steps Forward
Labs rarely run into trouble by sticking close to primary sources and always double-checking any chemical data before use. Standardizing data sheets, keeping a cross-check list, and building habits around careful weighing all make a difference. Anyone working with niche salts like tetraethylammonium trifluoromethanesulfonate counts on accuracy at every step—circumventing mistakes long before results get written up or published. Attention to detail in basic numbers enables creativity and confidence in the tough stuff that follows.
Understanding the Stakes
Tetraethylammonium trifluoromethanesulfonate shows up in plenty of labs where researchers work with ionic liquids or tinker with electrochemistry. Folks handling it often focus on how pure their sample stays or whether moisture creeps into bottles. Storage isn’t ever just an afterthought. One leaky bottle, a bit of humidity, and you’ve got a ruined experiment—or worse, serious safety headaches.
Why Storage Choices Matter
I’ve — like plenty of researchers — seen what happens when someone tosses a specialty salt on the back shelf without thinking twice. Tetraethylammonium trifluoromethanesulfonate usually comes as a solid, but it’s got a weak spot: moisture. The salt is notoriously hygroscopic. Water in the air doesn’t just make it clump up. That same water can trigger reactions or shift the chemical’s properties. I once watched someone scoop a clumpy mess into a reaction vial, wondering why nothing matched expectations. Frustrating, but all too common if storage takes a backseat.
Keeping Things Dry and Secure
Desiccators play a huge part in keeping this salt safe. Not the old-school glass ones collecting dust—we’re talking desiccators with strong, fresh desiccant and tight seals. Whenever possible, glass bottles with good screw caps or solid PTFE liners step up protection. I always label containers the day I receive a new batch and check seals every time. Moisture slips in quietly, so skipping routine checks cheats no one but yourself.
Temperature and Light Are No Minor Details
Leaving a bottle out in the sun speeds up trouble, sometimes in a matter of days. Light and heat slowly break down the compound, so I push for dark, room-temperature cabinets. About 20–25°C keeps things simple, and I avoid storing this salt near radiators or in direct sunlight. I’ve seen plenty of folks push every chemical onto the same shelf, chasing convenience. Yet with this salt, clutter costs—cross-contamination or temp swings risk the whole lot.
Labeling and Handling: More Than Formalities
Storing tetraethylammonium trifluoromethanesulfonate doesn’t stop once it’s behind a door. Labels must always show date of receipt, lot number, and—if you start portioning it—dates for every new bottle. I’ve watched labs forget this step, leading to years-old, degraded batches floating around. Quick record-keeping saves money and headaches. Transferring the salt always requires dry spatulas; I keep a stash of the disposable kind and never double-dip.
PPE and Safety Habits
This chemical deserves standard gloves, safety glasses, and a lab coat. Even though most folks think “it’s just a salt,” accidents escalate if dust ends up in your eyes or is inhaled. Storage spots need easy access to spill kits and eyewash stations, even if the risk feels remote. Over time, these habits become muscle memory—clean workspace, double-checked labels, and a watchful eye for leaks.
Solutions for Real-World Labs
I’ve found that clear protocols stop most headaches before they start. A well-posted storage procedure and calcium chloride in desiccators help even the busiest lab techs. Digital inventory logs keep track of expiry and stock rotation. A regular schedule—monthly checks, cleaning, and audits—keeps everyone accountable. Good storage stops a lot of problems at the source and lets people focus on what matters most: results and safety.
Chemical Curiosity Meets Practical Reality
Most folks don’t come across tetraethylammonium trifluoromethanesulfonate outside a chemistry lab. To someone who has handled all sorts of reagents in small rooms with noisy fume hoods, there’s always a good reason to double-check a label before pouring something out. For this salt, a mouthful in every way, the big question is: how risky is it to your health and the safety of your work environment?
Breaking Down the Hazards
This chemical flies under the radar of major hazard lists. It doesn’t have the same red-flag reputation as mercury compounds or cyanides. Still, just because it’s obscure doesn’t mean you toss the lab gloves. Most materials with a trifluoromethanesulfonate group have a reputation for irritating skin and eyes, sometimes more. Ammonium salts can mess with nerve transmission if you inhale dust or accidentally get them on your skin. Some researchers put tetraethylammonium salts into animal models in early nerve studies specifically because they block certain channels in nerves. If it gets inside the body, it finds ways to disrupt the usual rhythm.
Safety data for this precise salt seem thin. Scientists and safety managers rely on similar compounds’ data when actual information runs short. This one gives off noxious fumes if it contacts strong acid or burns, so fire safety stays important. Direct contact might redden the skin, and inhaling dust could trigger coughing, even in healthy folks. That’s not rare, but it’s reason enough to respect it in every routine.
It’s Not a Monster, But Don’t Get Casual
People working with this chemical use common sense: gloves, splash goggles, clean benches, and a fan pulling grit away from your nose. NIOSH and OSHA haven’t flagged it with panic, but research into closely related salts raised eyebrows across labs. Even if official “toxic” labels haven’t landed, working in a cloud of this stuff—especially as powder—makes as much sense as chewing fiberglass.
Why Proper Control Still Matters
Labs get busy, and folks cut corners, especially when they read that something sits “low-toxicity” on some info-sheet. Chemical safety officers will tell you stories about careers cut short by dismissing warnings. Acute toxicity might not land you in the emergency room, but repeated exposures add up. The lungs and hands are the most at risk. Over the years, I’ve seen too many people skip goggles for “just a quick transfer.” One slip, and you end up with chemical burns or an irritating cough that ruins your weekend.
Solutions and Real-World Choices
Common sense isn’t common enough, which is why repeating safety drills helps. Order only the quantity you use up within a project, and don’t let powder accumulate around scales or benches. Store tightly sealed, away from heat and acids. Clean up spills right away—never trust that it’s “just a little.” Build habits, not just rules: glove up, use that fume hood, and treat every transfer as if you’re just one messy slip from a lesson learned the hard way.
If the lab can swap in a less mysterious material for training or low-stakes experiments, do it. For folks at home, there’s no good reason to ever stock this. Respect for the chemicals, not just fear mongering, keeps everyone safe. As research moves forward, we’ll know more about the long-term effects—and it’s smarter to err on the side of safety until then.
The Real-World Utility of a Quaternary Ammonium Salt
Tetraethylammonium trifluoromethanesulfonate (TEATf) turns up in laboratories and industrial settings for a reason—scientists look for salts that offer both stability and flexibility with solvents. After years inside research labs, I’ve seen that success or failure with a chemical like TEATf does not just depend on textbook information; the actual handling, preparation, and results hinge on how well it blends and dissolves in various liquids.
Why Solubility Dictates Suitability
This compound stands out because of its impressive knack for dissolving across a broad set of solvents. Water, polar organics like acetonitrile, dimethyl sulfoxide (DMSO), methanol, and ethanol all welcome TEATf with open arms. Practical chemistry becomes easier when you know your electrolyte salts won’t stubbornly refuse to dissolve and clog up your process.
The trifluoromethanesulfonate part gives TEATf a non-coordinating anion, which means it blends into polar environments without forming sticky complexes with metals or other ions. The tetraethylammonium cation, known for size and bulk, supports that willingness to interact with water and organics rather than fighting for a nonpolar escape. I’ve personally witnessed this when prepping stock solutions for electrochemical experiments; you pour in the TEATf, stir briefly, and you’ve got a crystal-clear solution.
Practical Applications Driven by Solubility
Nobody wants to waste hours attempting to dissolve a compound that only half-mixes, leaving traces at the bottom of the flask. In batteries—especially non-aqueous ones—TEATf serves as a supporting electrolyte because of its solubility in solvents like acetonitrile or propylene carbonate. The whole point of using this salt is to create a predictable environment for ions to move—if you can’t get a dissolved, homogenous solution, performance plummets, and results scatter.
Organic chemists rely on TEATf for phase transfer reactions since its solubility bridges the gap between water and organic liquids. Clean separations, easier extractions, reliable product yields—these all benefit from a salt that doesn’t gum up the works.
Challenges and What Can Make It Work Better
TEATf’s good fortune does have limits. Its fame in polar solvents doesn’t mean it jumps right into nonpolar worlds like hexane or toluene. Drop it into these environments, and you’ll find it stubborn, forming layers or residues, refusing to mix. Chemists trying to innovate in mixed systems must accept these boundaries—adjust the setup, swap solvents, or look for surfactant action if a truly nonpolar mix is the goal.
Scaling up experiments brings its own pain points. Batch-to-batch reproducibility ties directly to how thoroughly you dissolve your salt. Inconsistent stirring, low purity, old solvent—all these factors slow solubility and can trigger confusion when results shift without warning.
Finding Solutions in the Lab and Industry
A solid practice, based on experience, involves warming the solvent gently to nudge things along. Filtering solutions before use helps remove silicate dust or insolubles from long-term storage. Quality control matters; using analytical tools like NMR or conductivity checks saves heartache later, ensuring the salt really did blend instead of merely hiding particles.
If you want consistent, high-performance mixtures—whether for batteries or bench experiments—there’s no shortcut around investing effort upfront to understand your salt and its habits. TEATf stands as a reminder: success in chemistry rides on knowing not just theory, but the actual behavior of your materials under real conditions.