Tetramethylammonium Tosylate: Insights and Real-World Relevance
Origins and Historical Development
Chemists have spent decades searching for clean, reliable quaternary ammonium salts. Tetramethylammonium tosylate didn’t spring from nowhere. In the mid-20th century, labs in Europe and North America started to take apart the reaction profiles between aromatic sulfonates and trialkylamines, shaping a clearer picture of how cationic surfactants react in solution. As the development of phase-transfer catalysts picked up in the 1970s and 1980s, this salt began to catch more attention. It arrived as a practical, lower-melting alternative in many cases where people found traditional alkali metal tosylates too unwieldy. Synthetic chemists found a tool that could bridge polar and organic phases—something that’s been a sticking point in non-aqueous organic syntheses for years.
Product Overview
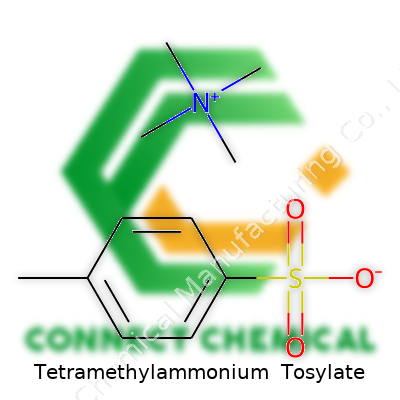
Tetramethylammonium tosylate combines the tetramethylammonium cation with the para-toluenesulfonate anion. This pairing produces a white crystalline powder—stable, easy to handle, and, for lab-scale work, less sensitive to water than some of its direct competitors. Suppliers cater to both research and small-scale manufacturing, often offering this salt in purities exceeding 98%. I’ve worked with batches kept in standard screw-top jars, labeled with nothing more than weight and batch number, yet the reproducibility still matched higher-profile, overpackaged brands. That consistency recognizes its role as a backbone material rather than a boutique compound.
Physical & Chemical Properties
On the bench, tetramethylammonium tosylate usually shows up as solid, odorless powder, bright white if free of mechanical grinding. The melting point sits in the range of 160–165°C, placing it out of reach for most ambient-temperature processes, but not so high as to require exotic heating methods. Its solubility remains a major strength: you’ll get good dissolution in water, DMSO, and methanol, while less polar solvents like diethyl ether leave most on the bottom of the flask. The salt doesn’t react with air, doesn’t absorb water as aggressively as alkali metal analogues, and stores best in cool, dry spaces—nothing fancy required. Chemically, it behaves as a source of both a non-nucleophilic base and a gentle tosylate ion, good for a slew of alkylation and methylation procedures.
Technical Specifications & Labeling
Product labels follow a straightforward format—common names, chemical formula (C12H19NO3S), and molecular weight (257.35 g/mol). Reliable suppliers guarantee a minimum of 98% purity, with residual water content rarely more than 0.5%. Impurities like formaldehyde or alternate sulfonates show up at trace levels, if at all. For GLP environments, standards demand more detailed documentation, but for most bench chemists, batch numbers and shelf life suffice. Handling instructions recommend gloves and goggles for anyone working in direct contact.
Preparation Method
The synthetic route starts with tetramethylammonium hydroxide and p-toluenesulfonic acid. Most lab methods lean into aqueous conditions. Tetramethylammonium hydroxide solution and a stoichiometric amount of p-toluenesulfonic acid come together in a beaker, giving off minor heat and sometimes a mild ammonia odor. Stirring leads to quick precipitation, followed by filtration and, more often than not, additional recrystallization from ethanol or water. On the industrial scale, chemists avoid any excess water, reduce the number of purification stages, and produce higher volumes with less loss, but the main steps remain unchanged. The accessibility of these starting materials means anyone with modest chemical training can set up the reaction.
Chemical Reactions & Modifications
Tetramethylammonium tosylate acts as more than just a passive spectator. Its quaternary ammonium core can serve as a phase-transfer catalyst, shifting ions across otherwise impenetrable solvent boundaries. The tosylate anion, being stable and weakly nucleophilic, rarely participates directly in side reactions aside from intentional sulfonation under severe conditions. In methylation protocols, the cation sometimes lands on aromatic or aliphatic systems, especially when pushed by strong bases or excess heat. Chemists in organometallic circles report its use as a counterion in anionic polymerizations and for neutralization processes after reaction completion. Under drastic conditions, the compound can undergo Hoffmann elimination, liberating trimethylamine, but in normal workflows, its chemical footprint stays predictable.
Synonyms & Product Names
In practice, chemists might see this salt listed as “TMA Tosylate,” “Tetramethylammonium p-toluenesulfonate,” or the formula abbreviation “TMA-OTs.” Some European catalogs prefer “N,N,N,N-Tetramethylammonium 4-methylbenzenesulfonate,” a mouthful few remember. Despite the naming chaos, experienced lab workers tend to recognize “TMA Tosylate” fastest, and that's what most suppliers print on bottles or online listings.
Safety & Operational Standards
Working with tetramethylammonium tosylate usually involves standard glove-and-goggle protocols—no need for elaborate ventilation unless you're weighing out several kilos. Skin and eye contact could cause irritation, mostly from the sulfonic acid residue or stray dust. Ingesting the compound is a bad idea, as quaternary ammonium salts disrupt nerve function; this risk sits squarely in the “don’t eat your chemicals” common sense realm. I’ve never encountered a workplace incident where this salt did more than sting an eye or irritate a hand, but the MSDS will stress the importance of proper disposal and spill management due to long-term aquatic toxicity for some ammonium salts. In regulated facilities, procedures place the container in defined secondary containment, and waste streams keep this compound well away from incompatible halides and oxidizers.
Application Area
Researchers turn to tetramethylammonium tosylate for its phase-transfer properties, especially when working on biphasic systems that call for ion movement between water and an organic phase. In analytical chemistry, it helps adjust ionic strength and buffer pH in HPLC protocols—small shifts, but essential for reproducible separations. I’ve worked on methylation of phenols and nucleoside analogues, where traditional methylating agents are too aggressive; here, the salt acts as a softer tool, delivering alkyl groups at a slower, more controlled pace. Some polymer chemists grab this material as a tailored counterion, tuning the solubility and reactivity of growing chains in solution or bulk processes. Academic labs favor tetramethylammonium tosylate largely for its affordability and easy handling, keeping it stocked as a go-to reagent when more exotic options price themselves out of student budgets.
Research & Development
Ongoing research highlights new domains for tetramethylammonium tosylate. Teams in Japan and the US have tested its efficacy in green chemistry—using water or ethanol as a reaction medium, banking on its solubility and mild cationic character to ease difficult substitutions. A few years back, a group in Germany leveraged its properties to support the electrochemical synthesis of pharmaceuticals, seeking better yields and cleaner product profiles. Startups studying ionic liquids often include this compound as a benchmark, measuring conductivity, viscosity, and compatibility for solvent-free catalysis. The persistence of this salt in published research shows its adaptability, not just as a placeholder but as a platform for testing new chemistry techniques on moderate budgets.
Toxicity Research
Toxicologists have long debated the safety of quaternary ammonium salts, especially those like tetramethylammonium with small, mobile cations. Acute exposure tends to cause mild irritation in mammals, though high doses bring about tremors, agitation, or respiratory difficulty due to interference with neurotransmitter pathways. In aquatic environments, persistent release could challenge the health of fish and invertebrates, prompting regulators to tighten disposal guidelines around ammonium salts in more eco-sensitive zones. Chronic exposure data remain sparse, as most users handle the compound in slow, careful protocols—gloved, goggled, and often under fume hoods. With new regulations coming into play across Europe and North America, manufacturers find themselves updating documentation and safety sheets far more often than ten years ago.
Future Prospects
As the chemical industry adapts to mounting regulations and shifting supply chains, compounds like tetramethylammonium tosylate, which promise safe handling and straightforward synthesis, have a good shot at wider adoption. Synthetic chemists look for salts that are easy to purify and recycle. Environmental pressures encourage process engineers to design shorter solvent cycles and tighter waste management—traits that favor salts stable in water and easy to recover. In labs where single-step methylation or phase transfer still bottlenecks innovation, incremental advances destined for pilot-scale production draw on old standbys like tetramethylammonium tosylate. With new generations of chemists prioritizing green chemistry, the need for gentle, predictable reagents will likely push this salt to stay in heavy rotation, provided manufacturers keep up with purity certification, safety training, and transparent sourcing practices.
Looking Past the Complex Name
Tetramethylammonium tosylate might sound like something you don’t want to spill on your kitchen floor. Chemists know it as a salt—one used in the lab for a pretty good range of things. It’s a tool, not a finished product you’ll find on store shelves, but it plays a behind-the-scenes role in shaping everything from pharmaceutical breakthroughs to advances in materials science.
Why Chemists Reach for Tetramethylammonium Tosylate
I remember my graduate lab days, always juggling reagents that required careful handling. This one stands out for a few reasons. Its main draw comes from the positively charged tetramethylammonium ion paired up with a tosylate group, which adds stability and changes how certain reactions behave. Chemists favor it in reactions where swapping out bits of molecules (known as “phase-transfer catalysis”) unlocks new possibilities.
In organic synthesis, it often takes the spotlight as a phase-transfer catalyst. That means it helps water-loving and oil-loving molecules get together—basically, acting as an interpreter when standard solvents don’t cut it. Without that bridge, some reactions would drag on or never even happen.
Shaping Pharmaceuticals and Materials
Drug research labs often rely on creating new compounds quickly and cleanly. Tetramethylammonium tosylate steps up as a helper in those syntheses. Its role can cut the number of steps scientists have to take, which matters when you only have one shot at making a tricky compound for a rare disease study. Time and resource savings translate directly into getting medicines to patients sooner.
Its influence doesn’t end with chemicals that heal—materials scientists have put it to work developing polymers and specialty materials. That work supports everything from better electronics to smarter packaging solutions. Bringing new materials online fast demands precise building blocks, and reliable reagents make it possible.
Environmental and Safety Considerations
Like any chemical, tetramethylammonium tosylate requires careful handling. Its environmental safety profile means labs working with it need solid disposal plans and respect for local rules. That’s the tradeoff each new tool brings: more options in the lab, but also more responsibility to the environment and fellow workers.
Companies and universities now invest more money in greener alternatives and safer protocols. Teaching lab students how to measure, store, and clean up after such chemicals can lower risks. As someone who’s cleaned more than my share of glassware, I know good practices start with respect for both the science and the hazards.
Room for Improvement
Tetramethylammonium tosylate isn’t perfect. Scientists keep looking for alternatives with better environmental profiles or that work just as well with fewer side-products. Open data sharing in journals now speeds up the search for these answers, which pushes the industry toward safer, more sustainable options.
Until that shift happens, the compound keeps earning its spot in the chemist’s lineup. Clear communication about its benefits and risks helps both experienced researchers and newcomers get the best from what it offers—while keeping an eye on what comes next.
Understanding the Components
Tetramethylammonium tosylate carries the chemical formula C12H19NO3S. This white crystalline salt combines two important pieces: the tetramethylammonium ion ((CH3)4N+) and the tosylate anion (C7H7SO3-). In the textbook, the formula often appears as (CH3)4N⁺ C7H7SO3⁻, showing both entities. It builds off two well-studied chemistry staples, both with their own sets of uses and quirks in the lab.
The Science Behind the Formula
Knowing the formula isn’t all about passing an exam or filling out a safety sheet. Scientists and everyday lab workers care about the composition because it shapes behavior — everything from solubility and stability to toxicity. Anyone who has spent afternoons measuring out reagents knows that ignoring the subtle details in a chemical’s structure can turn a routine experiment into a headache.
The tetramethylammonium ion brings four methyl groups sprouting from one nitrogen atom, a chunky, positively charged moiety. This “bulky” structure resists breaking down, giving it strength in organic reactions, especially in phase-transfer catalysis and as a base in organic syntheses.
The tosylate part stands for the para-toluenesulfonate group. Chemists often see tosylates as “leaving groups,” meaning they hop off molecules during reactions. Anyone who has tried to swap out a group in a molecule knows the stability and size of the tosylate anion make things possible that other groups could never pull off.
Why This Salt Matters
I remember a research project that hit a wall simply because the base we picked kept decomposing. We needed something tough but gentle in the right ways. Tetramethylammonium tosylate checked those boxes. Its bulk kept side reactions down, while the tosylate helped the chemistry run smoother. This salt finds its way into all sorts of places: organic synthesis, ionic liquids, and research on green solvents.
In industrial and pharmaceutical labs, people turn to this salt as a means to speed up reactions without hazardous metals or extremely strong acids. The formula tells scientists what’s possible and warns against mixing it with certain chemicals, because the sulfonate groups don’t play well with everything.
Researchers have kept close eyes on the potential toxicity. Tetramethylammonium-based salts have reputations for being neurotoxic in higher doses, so safe handling stays a priority. Wearing gloves, keeping benches clean, and having good ventilation aren’t just checklist items—they come from hard lessons learned by chemists over many years. Safety data for this salt lists a low LD50, with rapid death in animal models. This isn’t just trivia; it’s a warning that echoes in every organized, well-run lab.
Looking Ahead: Smarter, Safer Chemistry
With more attention on sustainability and green chemistry, researchers ask whether salts like tetramethylammonium tosylate serve as safer, reusable agents or if the toxicity risk is too great. Some teams are exploring biodegradable alternatives, and others are trying to make the best use of what exists by minimizing waste and improving recycling processes.
The chemical formula is more than a label — it tells a story about risks, rewards, and a path toward safer lab work. Knowing those few “letters and numbers” keeps experiments on track, workers safe, and science moving forward.
Why Storage of Tetramethylammonium Tosylate Matters
Tetramethylammonium Tosylate delivers valuable results in many labs. One thing that often gets overlooked is how storage decisions make or break its usefulness, as well as the safe operation of a workspace. From my time managing mid-sized teaching labs, I’ve seen what goes wrong when the basics of storage aren’t respected. One error or a shortcut can turn an otherwise reliable shelf chemical into a safety risk or a cause of experiment failure.
Tetramethylammonium Tosylate, an organic salt, reacts to moisture, light, and temperature swings. If you keep using the same jar for months and ignore humidity or exposure, you risk degrading the compound. The results show up as unreliable reactions or cloudy mixtures.
Keeping Moisture Out
This chemical pulls in water from the air. Once that happens, you’ll notice caking, clumping, or even a slight color change. Humidity may reduce its effectiveness in chemical reactions, introducing an unpredictable variable. Silica gel packets—those little drying agents—go a long way. An airtight, well-sealed amber glass bottle with fresh desiccant inside usually does the trick. Avoid plastic bottles. Over time, solvents can leach out from certain plastics and the container may warp, opening the door for more moisture.
I once worked in a lab that kept Tetramethylammonium Tosylate in open glass vials for convenience. Within a week, the chemical’s color and texture changed. After that incident, we switched to screw-cap bottles with thick rubber gaskets and kept a close eye on the moisture indicators inside the cabinet. This cut down on waste and ensured consistent reaction results.
Light and Temperature Considerations
Direct sunlight and open bench tops speed up degradation. While not the most light-sensitive chemical around, it slowly ages in clear light, which can alter purity and performance. Store the bottle in an opaque container inside a closed cabinet. Cold storage is not always necessary, but room temperature—steady and not sweltering—serves most labs well. Avoid extremes, since high temperatures might accelerate decomposition or release volatile residues, raising health concerns.
At a university job, I found a bottle kept next to a heat vent. That jar proved nearly useless for analysis after just a few months; every attempt to use it produced off results. Heat exposure creeps up on you, so place containers away from radiators, ovens, or sunny window ledges.
Labeling and Segregation
Nothing leads to costly mix-ups faster than vague labeling. Write full chemical names, hazard warnings, and the date of receipt on every bottle. Store away from acids and strong oxidizers. Mixing storage spaces or allowing cross contamination leads to risky, unexpected reactions. A strict routine for checking old bottles keeps surprises at bay.
Potential Solutions for Common Storage Problems
Investing in climate-controlled dry cabinets solves many headaches up front, especially for labs in humid regions. Digital humidity sensors give an extra layer of security. If budgets are tight, regular manual checks and replacing desiccants every month keeps most problems in check.
Training staff on proper closure, handling, and housekeeping tasks ensures chemicals like Tetramethylammonium Tosylate perform consistently. Good habits work better than expensive fixes in the long run. Many of these practices fit into established lab SOPs, and with a little attention, prevent many headaches down the road.
What We Know About Its Safety
Tetramethylammonium tosylate finds regular use in chemical labs for all sorts of reactions. With a formula combining tetramethylammonium, a well-known quaternary ammonium compound, and tosylate, this salt doesn't sit in most households, so direct exposure isn’t on most people's radar. Still, anyone who works with it, whether in research or industry, faces a basic question: does this compound bring significant health risks?
Understanding Its Toxicity
Tetramethylammonium ions carry a reputation. Some chemicals in this class pack quite a punch, proving toxic to nerves and muscles. Inhalation or swallowing brings on symptoms—think muscle weakness or, with higher doses, a breakdown in normal nerve function. Not many folks talk about this outside the lab, but the potential for harm lingers if someone gets careless.
Studies on the tosylate variant don't fill many books, but the main risk hails from its tetramethylammonium part. Poisoning cases, rare as they are, have produced seizures, trouble breathing, and in high enough doses, heart problems. Animal studies echo these effects. Data from the US National Library of Medicine backs this up, showing a median lethal dose (LD50) for animals stays relatively low. No one flirts with tetramethylammonium compounds outside professional circles for this reason.
Lab Safety Still Matters
Every chemist knows gloves, goggles, and a fume hood—the basics for handling stuff like this. Accidents happen, though. Even a seasoned expert forgets or rushes sometimes. Spills or splashes can turn into skin or eye burns, as some quaternary ammonium salts irritate tissues. Inhalation can mean lung and airway discomfort. Getting enough on your skin or in your lungs won't just stop at irritation. Emergency rooms worldwide handle unlucky cases every year.
In my own experience, I remember seeing a grad student faint in the fume hood after an unlabeled bottle leaked vapors. Afterward, stricter labeling habits and stricter buddy systems became the norm in that lab. Medical staff struggled to find information at first because not every variant, like the tosylate salt, gets well-documented toxicity records. It turned into a big reminder: never count on any ammonium compound being harmless.
Regulation and Responsibility
Tetramethylammonium tosylate doesn't show up in most government hazard lists, but similar compounds land on stricter registers. The problem isn't lack of data—it’s that many labs run with just-enough rules. Strong chemical safety programs, more thorough training, and easy access to full Safety Data Sheets (SDS) scale up protection. In chemical supply or research, better communication and regular sharing of incident stories make a major difference.
What Needs Fixing
Not every institution budgets for improved ventilation or more frequent safety drills. Some rely more on luck than preparation. Reinvesting in good engineering controls comes with real payoffs: lives stay safer, and accidents slow down. Digital tracking of chemical stocks, tighter access procedures, and timely replacement of old PPE also bring change.
Open conversations about risks—between supervisors, new hires, and support staff—goes further than paperwork. People need clear, real-life examples about what happens if they fumble a transfer or forget a mask. The minute the risks of compounds like tetramethylammonium tosylate go ignored, mistakes grow more costly.
Final Thoughts
Tetramethylammonium tosylate may look like an ordinary white solid. Under the wrong hands or in the wrong situation, it shows its dangerous side. Reliability and vigilance in chemical handling keep workers and labs from making tonight’s headlines.
Chemical Curiosity and Everyday Lab Work
Sometimes a single chemical says a lot about how science advances, and tetramethylammonium tosylate fits this scene. As someone who's often found peering at flasks and wondering if something will actually dissolve, questions about solubility go beyond textbook numbers. They matter at the bench, where unexpected results eat up time and energy.
What Happens When You Mix It With Water?
Put tetramethylammonium tosylate into water, and it dissolves pretty easily. The data shows its solubility lands above 100 grams per liter at room temperature. Most researchers and catalog manufacturers describe it as “very soluble” in water. You pour it in, stir, and without much drama, it turns clear before your eyes.
I've worked with salts that clump, haze, or float woodchip-like at the surface, so watching this one disappear into solution is a real relief. No heating or coaxing required. That kind of reliable behavior simplifies things for many chemical syntheses, phase-transfer reactions, and even some biochemical applications.
Why Does Easy Dissolving Matter?
A high solubility like this means fewer headaches. Water’s cheap, nontoxic, and always handy in a lab or production plant. No need to spring for exotic solvents or engineer strange workarounds. For folks running reactions or preparing buffers, soluble reagents keep messes and delays in check.
From my own experience, nothing saps enthusiasm for a project like a stubborn salt. Delays mount, and your concentrations wobble. Tetramethylammonium tosylate saves time by dissolving fast, letting chemists focus on the science instead of wrestling with stubborn solids.
What Makes It So Soluble?
Tetramethylammonium comes as a small, charged ion, paired with a bulky tosylate counterion. That structure encourages water molecules to surround and separate the ions easily. The absence of complex hydrogen bonds or large hydrophobic groups removes barriers that would slow dissolving.
Textbooks and research papers back this up. Sigma-Aldrich and TCI both confirm its strong affinity for water. Peer-reviewed literature, like a Journal of Physical Chemistry B paper from 2019, describes this salt as fully dissolving around 25°C with no reported limits at usual concentrations.
Concerns and Opportunities in the Lab
People sometimes forget that just because a salt dissolves, that doesn't mean the job is done. High solubility can lead to over-concentration, where reaction outcomes shift in hard-to-read ways. Old glassware or odd impurities can lead to precipitation, so accurate measurements and a clean flask always pay off. If quality control matters in your work, double-check with fresh solution prep before running expensive or time-intensive steps.
Wastewater streams from labs or plants also deserve thought. Highly soluble quaternary ammonium compounds can persist in the environment, so anyone working at scale should plan on responsible disposal.
Moving Forward: Beyond the Formula
Tetramethylammonium tosylate’s high solubility in water isn’t just a chemical fact—it’s a small gift to researchers who value reliability. If regulatory reviews, environmental safety, or new green technologies matter to your work, solubility turns from a yes-or-no question into the start of a bigger conversation. As more chemists and engineers hunt for greener and more practical solutions, evaluating the basics like this salt’s behavior in water makes pragmatic science possible.