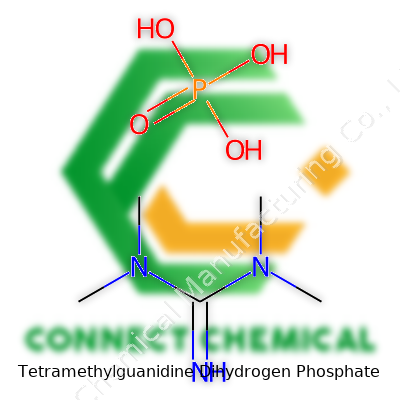
Tetramethylguanidine Dihydrogen Phosphate: Down-to-Earth Insights
Historical Development
Looking back to the history of organic synthesis, chemists in the post-war era often searched for better catalysts and safer, cleaner reagents. Tetramethylguanidine (TMG) popped up as a strong, non-nucleophilic base. As chemistry branched deeper into green approaches, researchers wanted to tame TMG’s basic power with safer counterions. That led to the pairing with dihydrogen phosphate, a well-understood, relatively benign anion. Early patents and scattered academic notes trace the salt’s earliest days to labs trying to mix efficient catalysis with environmental caution. Over time, chemists in both academia and industry found out that the salt delivered the best of both worlds: strong organic base character without the unpredictable reactivity that messy mineral bases could throw into a reaction.
Product Overview
Tetramethylguanidine dihydrogen phosphate gives chemists a handy solid that acts as a phase-transfer catalyst and base, but doesn’t carry the fire hazards or corrosive punch some other bases deliver. Its formulation, as a combination of a guanidine derivative and a phosphate, bridges the gap between traditional strong bases and more specialized reagents suited for modern green chemistry. Labs that once relied on liquid, smelly or caustic substances now store this white, crystalline powder in jars for safer, more predictable results. Process chemists appreciate one thing most: the salts’ shelf-stability and low volatility make storage and transport less of a headache.
Physical & Chemical Properties
The salt usually appears as a finely divided white solid, hygroscopic enough that you notice if you leave the bottle open too long. It dissolves well in water and polar organics—think methanol, ethanol, and DMSO. Its melting point sits above ambient but not too high, so it doesn’t clump from mild heat. The compound’s real value stems from its strong basicity. With a pKa nudging above 13 once liberated, the salt manages to push forward alkylations, esterifications, and other base-catalyzed steps. You also see good thermal stability, so it doesn’t break down in most lab ovens or reactors under routine use.
Technical Specifications & Labeling
Manufacturers provide bulk and lab-scale packaging, usually sealed tight to lock out moisture. Typical purity runs above 98%. Labels show the CAS number, batch stoichiometry, and minimal hazard warnings, since this compound doesn’t burn skin like sodium hydroxide. For the folks running stricter documentation, batch-specific certificates usually provide details on water content, heavy metals, and residual solvent traces, since those could trip up sensitive synthesis routes or regulatory filings. Shipping regulations tag it as non-hazardous, which cuts compliance red tape for international research and commercial transit.
Preparation Method
Labs prepare TMG dihydrogen phosphate by neutralizing tetramethylguanidine with phosphoric acid. The reaction is simple on paper: add TMG solution to slow-stirred, ice-cooled phosphoric acid. You’ll see immediate salt precipitation or thickening, since the product falls out in some solvents. Generations of synthetic chemists know to filter, wash, and dry for a high-purity product. Scale-up protocols rely on mild temperatures and controlled addition rates, ensuring each crystal comes out free from unwanted phosphate byproducts or partially protonated guanidines. The best setups control water content tightly, since too much moisture can impact downstream reactivity and handling.
Chemical Reactions & Modifications
TMG dihydrogen phosphate joins a family of phase-transfer catalysts and strong, non-metallic bases. It stands out by working in both aqueous and mixed solvent systems. The guanidine base ionizes and then pushes tough reactions over the edge—esters from carboxylic acids and alcohols, Michael reactions, and alkylations of relatively weak acids or nucleophiles. Researchers sometimes tweak the salt with co-catalysts or add small tweaks to the guanidine core, aiming for activity tailored to large-volume industrial runs. In my experience, the phosphate counterion sometimes gets swapped for more unusual anions in bespoke synthesis routes, especially where solubility control matters, but for broad use, the dihydrogen phosphate flavor holds its own in most mechanistic studies and production campaigns.
Synonyms & Product Names
Across catalogs and publications, you’ll meet this compound by a few aliases: TMG·H2PO4, N,N,N’,N’-Tetramethylguanidine dihydrogen phosphate, and TMG phosphate salt. Some suppliers stick with abbreviations, while regulatory filings spell it out, so it pays to double-check chemical identifiers before starting a procurement order. Despite the label changes, the chemistry and safety factors line up unchanged—it’s one of those rare cases where trade names don’t hide pitfalls or major differences in formulation.
Safety & Operational Standards
The shift from harsh mineral bases to TMG dihydrogen phosphate shrinks risks in both small-lab and plant environments. You don’t see the same caustic burns or inhalation hazards that plague older bases. Personal protective equipment means gloves and glasses, not full-face shields. Spills clean up with standard lab absorbents. Waste handling perks up too, thanks to lower aquatic and inhalation toxicity compared to heavy metal or strong acid alternatives. My own years in pilot-scale setups showed that using the salt sidesteps lots of headaches in reactor maintenance and floor cleanup, mostly because the product’s lower reactivity means less corrosive breakdown of equipment seals and pumps.
Application Area
The salt fits comfortably into medicinal chemistry, polymer science, and academic research. Drug firms favor it for straightforward amide couplings and protecting-group removals, especially where traces of harsh alkali cause more trouble than they solve. In plastics R&D, it speeds up reactions that are otherwise slow or incomplete with weaker bases. For fine chemicals, it helps scale up reactions from the bench to the pilot plant by combining strong base power with lower risk to staff and infrastructure. Environmental researchers have an eye on it too: the salt’s low aquatic toxicity lets more labs meet discharge requirements.
Research & Development
Interest keeps growing, especially where labs want cleaner, sustainable approaches. Recent articles explore tweaks to the guanidine core in pursuit of even more specific catalysis profiles. For instance, researchers probe how counterions affect not just catalytic rates but also ease of product isolation or purity in finished pharmaceuticals. In some university projects, TMG dihydrogen phosphate takes center stage as a base for asymmetric synthesis, which can mean fewer steps to a purer final active ingredient. Fields like electrochemistry and materials science keep probing for greener reagents able to handle charging, doping, or surface-modification uses.
Toxicity Research
Toxicologists appreciate TMG dihydrogen phosphate’s profile compared to the raw amine or most mineral bases. Rodent and cell-culture studies point to low acute toxicity, with most issues relating to eye and respiratory irritation at higher exposures. Chronic studies look better than many competitors, though researchers still monitor for subtle effects that could crop up in cumulative exposure scenarios. Wastewater and environmental assessments show quick breakdown and no significant accumulation in aquatic environments, a big plus for labs seeking certifications or greener reputation. There’s ongoing work in the industry to monitor long-term occupational exposure, although the decades since its introduction show only modest risk.
Future Prospects
Looking ahead, TMG dihydrogen phosphate seems ready to claim space in green chemistry and industrial scale-up. Academic labs keep finding new ways to use the salt in catalytic cycles or as part of designer ionic systems. Manufacturers push for larger production, since the switch from older bases means safer working conditions and easier waste handling. I expect automation and continuous-flow chemistry to step up, with TMG dihydrogen phosphate acting as a modular, predictable facilitator for tough reactions. Regulatory agencies may soon adopt explicit frameworks around these compounds, streamlining approvals for new drugs and specialty chemicals. The ongoing focus on workplace and product safety will nudge even more organizations to prefer salts that pack chemical punch without carrying steep health or environment costs.
Digging Into Tetramethylguanidine Dihydrogen Phosphate
Out in the real world, most folks never hear about tetramethylguanidine dihydrogen phosphate (TMG DHP). Yet in the busy backrooms of chemical labs, this compound draws serious attention. TMG DHP balances two key features: it acts as a strong base and brings in phosphate ions. Being both basic and acidic at the same time makes it useful for researchers who want fine control during tough chemical reactions.
Transforming Chemical Manufacturing
In pharmaceutical labs, synthesis needs sharp tools. Chemists reach for TMG DHP when tackling reactions requiring a strong push—think condensation, alkylation, or even stubborn esterifications. You won’t see TMG DHP on a medicine bottle, but you will find its fingerprints all over the steps that build up or modify active pharmaceutical ingredients. As drug chemists chase better yields and cleaner reactions, TMG DHP saves waste and improves safety compared to older, harsher additives.
Boosting Innovation in Materials Science
Innovation doesn’t happen in a vacuum. TMG DHP joins the toolkits of materials scientists who want better polymers and fine-tune properties like stickiness, flexibility, or chemical resistance. It steps in as a catalyst—nudging molecules to link up and form new plastics or high-tech coatings. Compared with standard acids or amines, TMG DHP offers more stable reaction conditions, which cuts down on impurities. This matters when engineers design electronics and automotive parts where every bit of performance counts.
Green Chemistry’s Hidden Helper
With pollution and climate change pressing in, industry leaders search for chemical tools that don’t load up landfills or produce noxious fumes. TMG DHP appeals to green chemists who want to reduce waste at the source. This compound can help shrink the need for heavy metals or less-friendly catalysts. Less pollution upstream spares headaches for waste treatment and clean-up crews down the line.
What Makes TMG DHP Stand Out?
Not every base carries the strange combination of high reactivity and safety in air. TMG DHP avoids explosive behavior, unlike some alternatives. In my time handling chemical procurement for a university research group, reliable shelf stability always means fewer accidents and less expensive safety gear. Simple storage matters as much as breakthrough performance, and TMG DHP rarely brings surprises on either front.
Access and Cost
Any specialty compound faces two big hurdles: availability and price. TMG DHP isn’t something you pick up at the corner store. Specialty suppliers stock it in small batches, which sometimes drives cost above that of simpler acids or bases. Still, for precise reactions—especially where expensive raw materials are at stake—many researchers gladly pay a premium to avoid later problems. As demand for smarter, safer, and greener chemistry grows, so does the reach of TMG DHP across industries.
Pushing for a Safer, Cleaner Future
Every seemingly obscure chemical carries a story deeper than its formula. TMG DHP offers a way forward for research teams hoping to trim environmental impacts without sacrificing performance. Wider adoption depends on teaching new scientists where and how it works best. Sharing real-world results, supporting training programs, and prioritizing transparent safety data can spread its benefits even further. Chemistry shapes our gadgets, medicines, and even the parts in our cars—and tools like TMG DHP help that work get done better and cleaner.
Storage Conditions That Protect Both People and Product
Tetramethylguanidine Dihydrogen Phosphate sounds like a tongue-twister, but for people working with chemicals, it shows up as a reliable tool in various labs and industries. Anyone familiar with chemical storage knows sloppiness puts both staff and materials at risk. I’ve walked through enough labs to spot quick shortcuts, and they almost always lead to unnecessary stress. Safe storage creates a calmer and more productive environment. This compound, like many organophosphates, calls for a storage setup that keeps things stable and reduces chances of disaster.
What Happens If You Ignore the Rules?
Chemicals react to their surroundings. Tetramethylguanidine Dihydrogen Phosphate responds to heat, moisture, and air. Storing it by the window, chucking it on a shelf beside the sink, or ignoring the label leaves the workspace wide open to spills, fumes, or worse. Using personal experience, I remember a junior researcher stashing a similar salt under a fume hood, which was closest to their bench. It looked harmless — until condensation seeped into the open bottle, leading to clumping. That same week, the supply team didn’t catch a slow leak in the lid, which led to a sticky mess by Monday. Small oversights lead to bigger problems down the line, from contaminated stock to hazardous fumes.
Key Facts for Safe Storage
Chemicals like Tetramethylguanidine Dihydrogen Phosphate fit best in a cool, dry, well-ventilated dark cabinet, with sturdy shelves marked for chemicals only. Sunlight heats up closed cabinets and speeds up degradation. Moisture creeping in through a broken seal accelerates hydrolysis, which breaks down the material and can release irritating byproducts. I always recommend desiccators or well-sealed containers, especially for hygroscopic compounds. Using proper secondary containment, like a plastic tray, helps if a spill does happen.
Temperature swings stress out many reagents, including this one. Room temperature usually works, as long as it stays below thirty degrees Celsius and out of direct sunlight. Most lab managers keep these reagents away from radiant heaters and out of high-traffic areas. It's easy to forget, but stacking bottles creates a risk if one tips over, so one layer per shelf makes clean-up easier.
Labels and Inventory: More Than Just Regulations
Clear labels go beyond bureaucracy. They cut down on confusion, prevent cross-contamination, and speed up emergency responses. I label every bottle with its full name, the date it arrived, and any hazards. This habit saved hours during a surprise chemical audit, but, more importantly, it means no one grabs a look-alike bottle and causes an accident by mixing the wrong chemicals. Good record-keeping tracks how old material gets before it becomes unreliable or unsafe.
Building a Safety Culture
No single product makes or breaks chemical safety. Teams build safe habits. Setting up regular audits for humidity, temperature, and container integrity catches slow leaks or creeping moisture before they escalate. Training sessions, tailored to new staff before they touch chemicals, matter as much as any checklist. Remembering stories from accidents — and sharing them — reminds everyone that lab safety isn’t just about regulations, but about caring for coworkers and the research itself.
Upgrading Your Setup
Modern storage cabinets equipped with humidity sensors and reinforced doors reduce risk. Investing in chemical-resistant shelving pays off long-term by stopping corrosion from drips or leaks. Working directly with reliable chemical suppliers ensures fresh material and the most up-to-date safety sheets. As regulations evolve, so should your setup — something I’ve learned every year when new workplace safety guidelines roll in.
Ultimately, the safest storage for Tetramethylguanidine Dihydrogen Phosphate means keeping it dry, cool, well-sealed, and properly labeled. These aren’t just checkboxes for audits; they’re steps toward a safer, more efficient workplace where everyone can focus on their real work — not cleaning up after preventable messes.
Understanding Chemicals in the Real World
People rarely stop and think about the compounds found in a laboratory or chemical plant. Tetramethylguanidine dihydrogen phosphate sits in that crowd of unfamiliar names, yet it often sparks interest—and worry—due to its chemical makeup. Lifting the curtain on this compound means digging into both its properties and its potential impact on health and the environment.
Looking at the Hazards
Tetramethylguanidine, as a parent compound, acts as a strong organic base. Left unchecked, it eats through skin and mucous membranes, triggering irritation or burns. Publishers from PubChem, Sigma-Aldrich, and similar sources all pin its toxicity on how corrosive it feels—eyes sting, lungs burn, fingers redden. True, dihydrogen phosphate tames things a bit, offering stability. Even with this softer touch, risk remains. Pick up a bottle of tetramethylguanidine dihydrogen phosphate without gloves, breathe in the dust, or spill some on the lab bench, and real consequences follow.
I’ve seen chemists decked out in coats and goggles for a reason. Even if the toxicity isn’t catastrophic by acute standards, overconfidence with chemicals opens the door to skin rashes, sore throats, or breaks in concentration that put whole teams at risk. Chemical safety documents, including material safety data sheets, always call for protective gear—lab-tested gloves, fitted eyewear, and sturdy lab coats. Ignoring these basics—just because something’s not “the most dangerous option”—proves short-sighted.
Long-Term Concerns: Beyond the Obvious
A spill or accident creates an immediate headache. The longer worries tackle builds up over time. If a small company lacks proper storage or training, chronic exposure can start to chip away at health. Repeated skin contact dries and cracks the skin. Inhalation brings coughing fits or, in bad cases, minor lung damage. Eye contact leads to lasting irritation. Genuine worry grows from repeated, unprotected contact, especially for those new to lab work who think they can “just be careful.”
Not every chemical gets a proper spotlight. Tetramethylguanidine dihydrogen phosphate isn’t as infamous as mercury or cyanide, but its mix of corrosiveness and potential to damage tells a clear story. Some safety guidelines feel excessive until an accident lands someone in the emergency room with burns or breathing trouble. That thought sticks with people who’ve seen slipups firsthand.
Solid Steps Forward
Working with this chemical safely starts long before it touches glassware. Good habits blossom from solid training led by people who refuse shortcuts. A confident, experienced supervisor should walk new staff through safety protocols instead of handing them printed instructions. Any workspace ought to post clear reminders to wear gloves and goggles, label storage cabinets, and lock up dangerous items when not in use.
Proper disposal also matters. Pouring leftover chemicals down the drain guarantees trouble down the line—corrosion in pipes, pollution in waterways, harm to aquatic life. Dedicated waste collection, careful documentation, and good housekeeping prevent most incidents before they begin. In a workplace that takes safety seriously, small warnings never fall on deaf ears.
Choose Respect, Not Fear
Tetramethylguanidine dihydrogen phosphate lines the shelves of labs and factories for a reason. Respect for its hazards, not fear, drives the safest results. Protective clothing, solid routines, and ongoing education offer the best shield against chemical hazards—now, and down the road.
Digging into the Details
Tetramethylguanidine dihydrogen phosphate sounds like a mouthful, but it’s a compound that researchers encounter when exploring organocatalysis, specialty synthesis, and advanced chemical processes. Understanding its basic numbers—chemical formula and molecular weight—matters in labs every day and pushes research forward. For those who spend time in a lab, double-checking a compound’s specs before mixing, measuring, or scaling up always feels like second nature.
Getting Precise: Chemical Formula
This salt forms from a union of tetramethylguanidine, a strong organic base, and dihydrogen phosphate, an acid anion. Tetramethylguanidine has the formula C5H13N3. On the other side, dihydrogen phosphate comes across as H2PO4-. Putting these together, their 1:1 combination leads to a molecular formula of C5H15N3O4P. Within this structure, you find all the basic organic elements—carbon, hydrogen, and nitrogen—blended with oxygen and phosphorus, a combination seen often in organophosphate chemistry.
The Math behind the Weight
Molecular weight influences dosing, handling, and both lab and industrial scales of chemical usage. Calculating the molecular weight for this compound leans on the atomic masses of its elements:
- Carbon (C): 5 x 12.01 = 60.05
- Hydrogen (H): 15 x 1.008 = 15.12
- Nitrogen (N): 3 x 14.01 = 42.03
- Oxygen (O): 4 x 16.00 = 64.00
- Phosphorus (P): 1 x 30.97 = 30.97
Summing those numbers gives a molecular weight of 212.17 g/mol for tetramethylguanidine dihydrogen phosphate. That figure guides chemists looking to prepare solutions or design synthesis routes, keeping experimental variables in check.
Why Getting the Numbers Right Matters
Back in grad school, nothing spoiled a whole batch of reactions faster than an error in one core calculation. Having a precise formula and a reliable molecular weight stops mistakes before they snowball, especially in synthesis. Small errors can waste expensive reagents or require repeating a long day’s work. This compound, used in organic transformations like guanidine-assisted catalysis and phosphate-related reactions, depends on trust in the data at hand. Publications rely on exact chemical information to ensure reproducibility. Without it, whole research projects could fall flat.
Headaches, Risks, and Room for Improvement
Mishandling a basic compound like tetramethylguanidine dihydrogen phosphate often leads to headaches in downtimes and missed opportunities. Many labs still double-check numbers manually, since some databases offer conflicting data or incorrect structural forms. This sparks a wider push for open, peer-reviewed chemical information repositories. Broad access to accurate data helps chemists worldwide, not just those lucky enough to work in well-funded institutions. Some digital databases, built by crowdsourcing and expert review, aim to clean up confusion, but they still run into the human error reality. Encouraging more collaboration between academic and industrial chemists could help accelerate updates, flag mistakes, and maintain quality. Those steps matter because foundational numbers like these don’t just sit in textbooks—they shape daily work, discoveries, and even safety on the bench.
Wrapping Up the Real World Impact
Tetramethylguanidine dihydrogen phosphate may sound obscure, but the lessons stack up. Precise, reliable chemical data isn’t just for writing journal articles or chasing intellectual curiosity. It’s about making sure every pipette pull, every weighed powder, and every hypothesis stands on a solid foundation—where accuracy builds better science for everyone in the field.
Why Proper Handling Really Matters
Tetramethylguanidine dihydrogen phosphate, a mouthful for sure, crops up in labs and industry pretty often. This chemical doesn’t scream danger on every label, but beneath the surface, risks pile up if folks let routine slip. The burn, the sting, the nasty cough from a careless whiff — this stuff won’t win any popularity contests in a chemistry class. No one sets out to hurt themselves or the planet, but accidents stack up fast if careless hands rush or forget basic respect for chemicals. Years of work around all kinds of reagents taught me that safety isn’t paranoia — it’s just common sense.
PPE and Smart Storage Go Hand in Hand
Daily lab life gets full of shortcuts, but cutting corners on safety gear rarely works out. With tetramethylguanidine dihydrogen phosphate, gloves built for chemical resistance shield the skin. Normal latex feels useless after a few splashes, so switching up to nitrile or neoprene keeps burns at bay. Eyes stay well protected behind wraparound goggles — even one tiny splash stings, and no quick rinse solves it. Fumes don’t mess around either, especially in badly ventilated corners. Working near a fume hood lets the air clear out instead of getting trapped where lungs don’t want it.
Chemicals that react with water or strong bases can build up heat or generate nasty byproducts. Keeping them sealed tight in labeled bottles, away from acids and other volatile organics, stops surprise reactions before they start. Secure storage racks, clear labels, and up-to-date inventories turn messy shelves into safe workspaces. It’s less about rules, more about teammates making sure no one else pays for a lazy choice.
Disposal: The Temptation to Cut Corners
Dumping leftover chemicals down the drain might seem easy, but the aftershocks linger in water systems. Sewage plants aren’t built for treats like this — they pass right through, sometimes turning into even nastier compounds downstream. This stuff doesn’t magically disappear, and local wildlife carries the cost, often far away from where it started. Dumpsters for “household waste” don’t fare better, since landfill leaks travel easily through rainwater.
University labs take disposal seriously and partner with trusted hazardous waste services for a reason. Collecting even small amounts in labeled waste jars, tracking volume, and logging handoffs keeps records tight and accidents few. Spills, even small ones, get treated like red-alerts: absorb with spill pads, followed by careful cleaning, and sealing away every rag or wipe in a chemical-resistant bag for disposal. Extra paperwork might slow the day down, but the cleanup from one bad spill spirals into days of lost time.
Training, Trust, and the Human Factor
Safe handling relies as much on trust among coworkers as it does on equipment. Anyone can grab goggles or read a label, but building a culture where others speak up takes time. Just last year, our lab replaced a bottle missing a proper label and updated everyone’s training sign-off — no punishment, just fixing a problem together. Trust grows in shared routines: checking labels twice, not letting old waste jars “rest” in a dark corner, asking about a smell before ignoring it.
The science won’t stand still, and new chemicals show up every season. Yet, the basics — pay attention, respect the risks, keep an eye on the end of the chain — stick around. Keeping safety at the front doesn’t make the work slower or boring. It builds spaces people come home from, every single day.