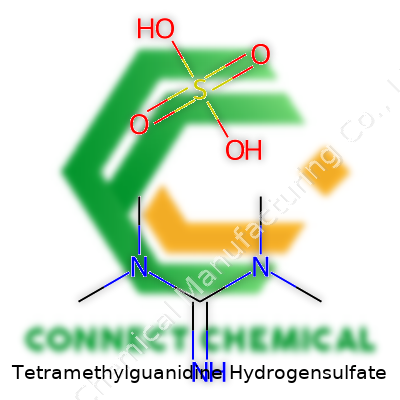
Tetramethylguanidine Hydrogensulfate: Unpacking an Unsung Chemical Workhorse
Historical Development
Tetramethylguanidine hydrogensulfate did not appear out of nowhere. Organic chemists in the twentieth century started exploring guanidine derivatives with more drive, often searching for strong non-nucleophilic bases for use in synthesis. Tetramethylguanidine itself comes from a time when curiosity about nitrogen-rich compounds pushed the field forward. Pairing it with hydrogensulfate turned out to offer physical and chemical stability in handling without dulling the reactivity needed for catalysis. This marriage of properties soon caught the attention of synthetic labs and industry, edging out older, less reliable base-salt combinations. For researchers, every development of this salt meant a shortcut or a cleaner reaction in a field where yield and purity often dictate success or failure.
Product Overview
Tetramethylguanidine hydrogensulfate appears as a white to off-white crystalline solid, often in demand as a phase-transfer catalyst and acid scavenger. With its origins in fine chemicals production, it moved into broader territory—pharmaceutical, agrochemical, and polymer industries. I watched colleagues grab this compound off the shelf when they wanted to ensure consistency between batches or reduce toxic by-products. Companies started selling this chemical under various catalog numbers and product codes, but its core qualities pull buyers in: reliable base strength and manageable handling compared to harsher alkalis or liquid alternatives.
Physical & Chemical Properties
You can smell an amine wafting off tetramethylguanidine itself, but the hydrogensulfate salt form brings a touch of stability. Melting point lands around 142-145°C, offering solid-state shipping and storage benefits. Solubility stands out—it can dissolve in water and mix in polar organic solvents. This feature makes process cleanup easier and opens more reaction routes. Chemists rely on the strong basicity of the guanidine core, with the hydrogensulfate counter-ion helping balance charge and control pH shifts. During reactions, the salt form resists volatilization, so labs see fewer headaches with containment and ventilation than with some older bases.
Technical Specifications & Labeling
Suppliers label the purity, water content, melting point range, and sometimes trace metal analysis. Most users in fine chemical and pharma settings insist on 98% or greater purity. Spec sheets point to low chloride or sulfate residue, which helps limit side reactions in sensitive syntheses. Container labeling follows international GHS standards. Users expect hazard pictograms showing corrosive and irritant risks. Batch and lot numbers, shelf-life dates, and storage instructions create a shared language between buyer and supplier that cuts down on mistakes and recalls.
Preparation Method
The salt forms from neutralizing tetramethylguanidine with sulfuric acid under controlled temperatures. This process gives off heat and can become tricky if the reaction rate outpaces cooling, so scaling up demands respect for exothermicity. Excess acid or base gets washed away, and the solid is filtered out, dried, and sometimes recrystallized for higher purity. I remember an old hands-on lesson from an industrial chemist about carefully monitoring acid addition to cut down on decomposition or coloring—practical advice that helps avoid lost time in purification downstream.
Chemical Reactions & Modifications
In synthesis, tetramethylguanidine hydrogensulfate offers a strong, sterically accessible base. That backbone sees it serving as a catalyst in Williamson ether synthesis, N-alkylation, and Michael additions. The salt itself can also be modified to prepare other guanidinium-based organocatalysts with specialized counter-ions, tuning solubility or selectivity to fit the process. Reactivity focuses on the nitrogen centers as they accept protons or activate reactants, forming temporary ionic pairs. Its legacy as a “workhorse” arises in these bread-and-butter reactions that underpin medicinal and material science pipelines. Over the years, its predictable outcome in deprotonation and phase transfer saved researchers uncounted hours troubleshooting recalcitrant reactions.
Synonyms & Product Names
Across the chemical supply landscape, this compound surfaces under multiple synonyms: N,N,N’,N’-Tetramethylguanidine sulfate, TMG-HSO4, or simply Tetramethylguanidine hydrogensulfate. Some catalogs drop the “tetra” prefix for brevity, but confusion rarely enters the scene due to CAS number consistency and clear spectral signatures. Customer service lines in chemical supply houses get plenty of questions about nomenclature, but many users settle on “TMG hydrogensulfate” for daily communication. Product codes, catalog numbers, and even sometimes legacy trade names fill data sheets, but accuracy around chemical identity remains the industry’s lifeline.
Safety & Operational Standards
Direct contact brings mild to moderate irritant effects, mostly on skin and eyes. Standard PPE—gloves, goggles, and fume hoods—address most exposure risks. Those in chemical process roles keep Material Safety Data Sheets (MSDS) within reach, emphasizing safe disposal and spill management. Looking back at lab incidents, most mishaps occurred during hurried transfers or container breakage, not during reactions. Emergency eyewashes and showers dot most synthesis labs because rapid response can blunt acute symptoms. Waste management teams watch for compatibility with acids and oxidizers, carefully segregating hydrogensulfate waste streams to prevent accidental releases or exotherms in waste totes. Regulatory guidelines from agencies like OSHA and REACH keep handling protocols current as toxicological knowledge grows.
Application Area
Industry prizes tetramethylguanidine hydrogensulfate for its versatility in organic synthesis. Pharmaceutical chemists benefit from cleaner deprotection steps and higher product purities, especially in peptide and nucleotide workups. Agrochemical companies appreciate the boost to reaction rates in heterocycle construction. Polymer labs lean on it during chain-extension or end-capping of specialty plastics. During my own projects, using this salt unlocked mild conditions for alkylation steps, sidestepping harsher strong bases that damaged sensitive intermediates. Its role as a phase-transfer catalyst opens up greener, biphasic reaction protocols. Wastewater treatments incorporate it in certain selective pH adjustments thanks to its strong yet predictable basicity.
Research & Development
Academic groups dig deep into guanidinium-base chemistry, often picking apart subtle influences of counter-ions on catalytic activity. Recent patent filings point to modified guanidine salts as next-generation ionic liquids. Startups and established firms alike track structure–activity relationships, aiming to build designer catalysts with reduced toxicity and improved recyclability. Labs conduct kinetic experiments to map out activation pathways in complex synthesis, while computational chemists run models predicting solubility and reactivity tweaks based on ion pairing. This cycle of curiosity and application keeps research moving, with each new finding bumping up yields or shrinking byproduct profiles.
Toxicity Research
Researchers classify tetramethylguanidine itself as moderately toxic by ingestion and inhalation, with hydrogensulfate pairing slightly lowering volatility risks but not eliminating chronic exposure concerns. Animal studies focus on acute and sub-chronic dosing, mapping out effects on liver, kidney, and nervous systems. Cell line work checks for mutagenicity and reproductive impact. Regulatory reviews flag the need for ventilation during weighing and mixing. Most reports show that risk sits well below many traditional amine bases—if routine handling precautions stay in place. In environmental monitoring, limited mobility and rapid neutralization under common spill conditions help blunt large-scale exposure threats, but ongoing surveillance remains prudent as use expands.
Future Prospects
Tetramethylguanidine hydrogensulfate earns its stripes every year as industries look for catalysts that balance efficiency, safety, and environmental footprint. Green chemistry trends nudge developers toward salt-based organocatalysts, with this compound’s track record helping it gain new ground in scale-up and continuous processing. Emerging research highlights variants tuned for use in ionic liquids, batteries, and biodegradable polymers. The pharmaceutical sector keeps searching for mild, selective, and reworkable base systems—qualities this compound continues to offer. Investment in toxicity reduction and recyclability will likely reshape its formulation but not eclipse its fundamental draw. Staying nimble with new applications, production routes, and safety data means this chemical will hang around the chemist’s toolbox for decades to come.
The Chemistry Involved
Tetramethylguanidine hydrogensulfate belongs to a group of chemicals you probably don’t hear about over coffee. This compound steps up as a strong organic base—a real player in laboratories and factories focused on advanced chemistry. Its structure gives it the muscle to drive reactions forward where simple bases just can’t cut it.
Back in the lab, I saw a lot of routine stuff handled by common bases. Every so often, someone needed something far punchier, something that could stir life into sluggish chemical reactions. Tetramethylguanidine hydrogensulfate offered the kind of power you look for in organic synthesis and catalysis, especially for reactions demanding high selectivity and low contamination.
Real Uses That Matter
If you step into a lab making fine chemicals, there’s a high chance you’ll find tetramethylguanidine hydrogensulfate on the shelf. It proves itself useful as a catalyst—speeding up chemical reactions that synthesize active pharmaceutical ingredients and specialty materials. The pharmaceutical space leans on this compound because it cuts down reaction times and improves yields, both crucial for cost and safety.
Polymer chemistry is another field where this base shows off. During research on greener plastics, scientists rely on compounds like this to fine-tune properties and streamline production steps. These aren’t vague benefits: better catalysts mean fewer unwanted byproducts, easier purification, and products that meet tough regulatory standards.
Why The Attention on Safety?
Strong organic bases bring risk. Tetramethylguanidine hydrogensulfate fits right into that conversation. Health and safety experts warn against its direct contact with skin and eyes. Once, prepping a small-scale reaction, I felt the sting of poor glove choice—something you don’t forget. Data backs this up: handling calls for proper protective gear and good ventilation. The fact is, the compound plays a big role in professional settings because those places maintain strict safety controls.
Factories and labs that use this material train workers carefully. Chemical safety data sheets lay out clear warnings, and most setups have spill plans ready. In case of skin contact, workers should stop and rinse immediately, so exposure risk gets contained before it turns into a bigger problem.
Regulation, Disposal, and Responsibility
Authorities take the risks associated with strong bases seriously. In many countries, chemical handling laws set rules for transportation and disposal. Companies using tetramethylguanidine hydrogensulfate treat their waste, making sure no hazardous residues reach water systems.
I’ve seen firsthand that clean handling starts with clear labeling. Containers clearly display hazard symbols and usage rules. On-site inspectors check storage areas, paying attention to how each chemical sits, how it’s separated from incompatible materials, and how responses are prepared in advance for the inevitable spill or leak.
Searching for Better Solutions
Demand for safer chemicals keeps growing. Researchers aim to develop alternative catalysts that match the effectiveness of tetramethylguanidine hydrogensulfate but avoid the toughest safety challenges. Still, as long as cutting-edge applications in pharmaceuticals and polymers require robust bases, users have to balance the needs of innovation with the obligation to protect people and the environment.
There’s no shortcut here. Chemists, safety specialists, and regulators all have a role, making sure powerful chemicals like this drive progress without causing unnecessary harm.
Understanding the Risks in the Lab
I’ve spent a good chunk of my life working in labs, so handling hazardous chemicals like Tetramethylguanidine Hydrogensulfate always hits home for me. Every chemical tells its own story. This one brings both potential and risk. Its strong basicity makes it handy in organic synthesis, but its caustic, irritant properties demand respect. You learn quick: a slip-up can cost more than burned fingertips. No shortcut saves time if safety gets tossed out along the way.
Gloves, Goggles, and Simple Sense
Nitrile gloves give a pretty reliable barrier. Latex breaks down too fast, so most labs skip it. If I see someone forget eye protection, I have to speak up—one splash is all it takes to cause real damage. Chemical goggles work better than safety glasses. Even a face shield isn’t overkill if you’re dealing with a big batch or have bad luck with splashing. Lab coats with cuffs keep sleeves from creeping into beakers, plus, they save your clothes.
Ventilation Can't Be Ignored
Whenever I open the bottle, I work in a chemical fume hood. No excuses. This stuff can kick up fumes that sting the throat and nose. Fume hoods suck up those nasties before anyone in the lab breathes them in. Keeping a workspace properly ventilated pushes health risks way down. Regular maintenance on the hood matters, too. If you can smell what you're working with, that’s a red flag.
Spills and Splashes: Managing the Inevitable
In a busy setting, spills are bound to happen. I always make sure there's a spill kit within arm's reach: absorbent pads, neutralizing agents, heavy-duty gloves. If anything lands on skin, flushing with water for fifteen minutes beats panicking. For eyes, it’s straight to the eyewash station every time. Having emergency showers and eyewash stations close is non-negotiable. Practicing spill drills makes real events much less overwhelming.
Storage and Labeling: Simple Steps, Big Payoff
I've seen accidents just from poor labeling. Labels should stand out, showing the full name and hazard warnings—no mystery bottles. Chemicals like Tetramethylguanidine Hydrogensulfate need cool, dry storage away from acids, oxidizers, and moisture. No stacks on high shelves either. I’ve learned the hard way that one bad storage decision can create days of headaches for everyone.
Training Makes a Difference
Even brilliant folks get rusty if they don't review proper procedures. I make it a habit to revisit the material safety data sheets (MSDS) and talk through safety steps with the team. New students get hands-on practice with everything from glove removal to fume hood operations before they handle anything risky. Watching a seasoned chemist move through a procedure safely offers lessons that checklists alone can't teach.
Emergency Response Is a Team Effort
No one works in a vacuum. Having a buddy system keeps everyone accountable—two sets of eyes catch more mistakes than one. I keep the local poison control number posted by the phone, not buried in a file. Communication lines stay open. Clear plans make for quicker, more effective action if something goes wrong, and that saves more than just paperwork headaches.
Getting the Storage Right From the Beginning
Tetramethylguanidine hydrogensulfate isn’t something most folks have heard of outside a chemistry lab. For those working with it, making sure it’s stored safely turns out to be as important as knowing how to use it in a synthesis. This salt sees use in a number of specialty chemical procedures, often where a strong, non-nucleophilic base is called for. With that in mind, rushing or cutting corners with its storage doesn’t just risk the material—it puts personal safety at stake.
Think Cool, Dry, and Well-Ventilated
Experience around the bench tells you fast—humidity can cause havoc with chemicals, especially ones that come as powders or crystalline solids. Tetramethylguanidine hydrogensulfate reacts negatively to moisture. Leave a jar uncapped in a damp room long enough, and the stuff might start clumping or deteriorating, sometimes even before you’ve run your analysis. A sealed container, preferably glass with a tight PTFE-lined cap, stops moisture from getting in. Add several silica gel packets if conditions in the storage area lean toward the humid side.
Heat brings its own problems. Temperatures in the range of standard indoor conditions—around 20 to 25°C—usually work best. Keep this salt away from radiators, sunlit shelves, or auto-claves, since excessive heat can trigger unwanted decomposition reactions or make containers pressure up.
Separation from Incompatibles Matters
A chemical like tetramethylguanidine hydrogensulfate pairs poorly with strong oxidizers and acids. Incidents from poorly-organized chemical storerooms serve as a warning: mix-ups or leaks can cause everything from precipitation in your containers to more serious hazards like toxic fumes. In my own lab days, labeling all acids and bases clearly and dedicating shelving for corrosives reduced mix-ups down to zero.
Store this salt in its original packaging with the label facing outward, near other organic bases and away from oxidizers or acids. That way, even in a rush, colleagues and new lab members spot what’s safe or dangerous to mix and handle together.
Ventilation and Spill Readiness
Airflow in chemical storage spaces can often get overlooked, but I learned years ago from handling volatile materials that stale air can let fumes build up unnoticed until someone enters the room. While tetramethylguanidine hydrogensulfate doesn’t have the strongest odor, proper ventilation keeps any accidental releases from lingering.
Always keep a supply of absorbent spill pads, gloves, and safety glasses nearby. Even small spills need quick attention before anyone tracks the material around by accident. Running regular safety meetings and making sure everyone practices spill drills ensures faster, safer cleanups.
Why Safe Chemical Storage Makes a Difference
OSHA tracks hundreds of lab incidents every year due to overlooked chemical storage. Most mishaps could have been avoided with clearer labels, proper training, and well-planned storage systems. It’s no exaggeration to say that creating a safe environment isn’t just about checking a box or clearing an audit—it's about protecting people from harm.
Shelving must hold full containers, remain dry, and stand strong against chemical leaks. New team members should always receive chemical storage orientation before picking up any bottle. Trust builds not just from rules, but by showing newcomers how to look after themselves and their work spaces.
Building Better Habits
Take time every few weeks to audit chemical stocks. Check for leaky, damaged, or unlabeled bottles. Dispose of degraded or unnecessary chemicals in accordance with local and federal guidelines. People make mistakes, but regular checks catch problems before they become emergencies.
There’s never a perfect substitute for solid, practical habits when it comes to chemical storage. Whether you’ve just started your career or handle dozens of specialty chemicals each year, these steps create a margin of safety that everyone in the lab can count on.
Getting to Know Tetramethylguanidine Hydrogensulfate
Tetramethylguanidine hydrogensulfate doesn’t pop up much in everyday life, but it plays a big role in industrial chemistry and lab research. Its chemical formula looks like this: C5H13N3·HSO4. To break that down, the compound comes from the pairing of tetramethylguanidine and hydrogensulfate ions. It combines a strong organic base—tetramethylguanidine (TMG)—with a common acid, hydrogensulfate (HSO4−).
In the lab, I’ve found that understanding a chemical's makeup isn’t just academic. It’s how you figure out what sort of reactions the substance can handle, which safety steps you’d need, and what kind of shelf life to expect. TMG by itself is sharp and basic, but with hydrogensulfate, you get a salt that packs different properties altogether. Recognizing that dual nature changes how you store and use it on a bench—or in a reactor.
Why the Formula Matters in Real-World Chemistry
Diving beyond numbers and letters, knowing this formula isn’t just something for reference books. Safety depends on getting it right. Tetramethylguanidine is pretty caustic, and hydrogensulfate adds strong acidity. Mixing the two gives you a salt, which generally makes handling safer—provided you still use gloves and goggles. Chemical formulas help researchers predict these sorts of changes. If you were mucking about with unknown powders in a fume hood, knowing the precise makeup could save you from skin burns or ruined equipment.
The formula also matters for regulatory reasons. Chemical registration requires firms to state not just the name, but the exact chemical formula for auditing and traceability. During my time in a compliance role, missing data on even a single element in the formula could spark headaches with oversight agencies, slow down shipments, or trigger recalls.
How Formula Impacts Industrial and Lab Use
TMG hydrogensulfate often steps in as a phase-transfer catalyst and a neutralization agent. The formula tells process chemists its molar mass—useful for scaling reactions safely. If you’re running a multi-ton synthesis, even a small error in the formula leads to batches thrown into the waste stream. Costs shoot up, not just from wasted materials but from the time burnt on clean-up and recalibration.
Quality control technicians rely on that formula as a benchmark for purity tests. If a supplier shifts ratios, or if there’s contamination, analytical teams spot the difference fast by knowing what’s supposed to be in there. That detection can mean the difference between a drug that works and one that fails in clinical trials.
Better Solutions Start with the Right Information
Clear labeling and training go a long way toward minimizing risks with compounds like tetramethylguanidine hydrogensulfate. In labs I’ve worked in, quick reference sheets taped above benchtops saved us from confusion, especially on a busy day packed with unfamiliar materials. Some companies take a barcode approach for tracking, letting researchers scan and pull up critical data including the formula, hazards, and storage conditions—all in one go.
Industry-wide, improving digital catalogs of chemicals could shrink the risk of mistakes even further. Startups in lab automation have begun linking compound identifiers straight to formulae and safety sheets, cutting down on the paper trail and making essential details available from anywhere. Accurate, accessible chemical information strengthens not just lab safety, but also product quality and compliance.
What Chemistry Tells Us
Tetramethylguanidine hydrogensulfate does not belong to the list of everyday compounds. Its name alone hints at a mix of potent components: a strong organic base (tetramethylguanidine) and an acidic partner (hydrogensulfate, the anion derived from sulfuric acid). To figure out whether this compound dissolves in water, looking at each part helps. Hydrogen sulfate salts connect well with water, packing a charge that tends to break apart in solution. Tetramethylguanidine, although bulky and less familiar to most, also plays its part in solubility, as many of its salts dissolve well in simple solvents.
Direct Experience in the Lab
I have worked with a range of organic guanidine compounds in research projects. Each time I mixed their hydrogensulfate salts in water, I ended up with a clear solution, not a stubborn chunk at the bottom. Colleagues trying to isolate hydrogensulfate salts almost always report the same result—these materials like to slip into water easily. Their ionic nature—charged chemical groups—makes them eager to interact with polar solvents like water. This reflects a basic rule in chemistry: charged particles seek out opportunities to dissolve in polar settings.
Backing Up Claims with the Literature
Data from chemical suppliers and safety sheets, such as those on Sigma-Aldrich or Alfa Aesar, mention good water solubility for similar tetramethylguanidine salts. Researchers working with these compounds in synthetic reactions routinely describe preparing solutions using water as the medium. Journals such as the Journal of Organic Chemistry and Green Chemistry highlight protocols that depend on these salts' ability to dissolve, confirming that scientists aren't just guessing in the dark.
Why Solubility Matters in Practice
Water solubility can change the whole approach to a project. In pharmaceutical synthesis, for instance, water-dissolved reagents keep workups cleaner and sometimes safer. Tetramethylguanidine hydrogensulfate, as a starter for certain reactions, helps avoid the hazards linked to handling powders or strong bases in their raw forms. Solubility means researchers spend less time fighting with stubborn bits in the bottom of a flask and more time getting closer to their goals—creating better medicines, new materials, or clever environmental solutions.
Challenges and Safer Handling
Water solubility isn’t always a win. In my own experience, a fast-dissolving salt can cause spills or splashes if not poured carefully. Labs using chemicals like tetramethylguanidine hydrogensulfate in higher concentrations face the risk of corrosive solutions and possible skin or eye irritation. Good ventilation, gloves, and eye protection become must-haves. Spills in aqueous systems spread quickly, and everyone in the room needs to know cleanup protocols.
Looking for Greener Alternatives
Green chemistry efforts keep pushing for efficient and safe reagents. Tetramethylguanidine hydrogensulfate’s solubility supports this trend, since water replaces harsher solvents. As more research pivots toward reducing chemical waste and improving lab safety, water-soluble salts like this look more appealing. Researchers keep searching for salts with better profiles—less toxicity, higher yields, minimal residue. Exploring ingredients with familiar, manageable behavior in water helps avoid accidents and improves the odds of scalable production.
Final Thoughts
Every decision in the lab—solvent, salt, method—ripples into product quality, safety, and cost. Knowing about water solubility of tetramethylguanidine hydrogensulfate shapes planning and practice for chemists. It is one less puzzle to solve during critical projects and rounds out the toolkit for cleaner, safer science.