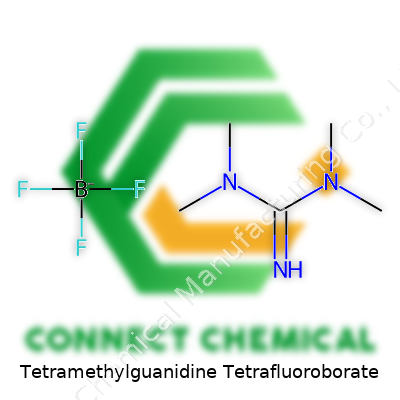
Tetramethylguanidine Tetrafluoroborate: A Closer Look at Its Role in Modern Chemistry
Historical Development
Chemists started paying attention to tetramethylguanidine (TMG) as early as the 1960s, looking to develop non-nucleophilic organic bases that could hold up in harsh environments. After fluoride and perchlorate salts saw widespread use, a hunger for strong, chemically stable ionic compounds led researchers to investigate new pairings. Tetrafluoroborate emerged as a reliable anion, showing low nucleophilicity and impressive thermal stability. The combination of TMG and tetrafluoroborate grew out of this search, fueled by the need for dry, easily handled basic salts in organic synthesis, especially when older bases like KOH or NaOH failed to give clean results.
Product Overview
Tetramethylguanidine tetrafluoroborate appears as a white crystalline powder, fairly easy to handle compared to other strong organic bases. Scientists often turn to this salt for processes where a non-aqueous, non-coordinating base is essential. Its presence in labs has grown alongside rising use in catalysis, ionic liquids, and specialty organic synthesis, where its unique blend of stability and reactivity means fewer side reactions and simpler purification.
Physical & Chemical Properties
You won’t find this compound melting or boiling at extremely low temperatures. Its melting point usually falls around 120-130°C, and its solubility profile gives it the edge in reactions that run in solvents like acetonitrile, dichloromethane, or DMF. The compound does not react vigorously with water but does show hydrolysis over time. TMG tetrafluoroborate’s low volatility and essentially odorless nature set it apart from many amine-based salts, making it far less irritating for everyday lab work. As a quaternary ammonium salt, its cation is strongly basic but the BF4- counterion helps reduce unwanted side reactions. The crystalline form packs well, stays stable under most lab conditions, and resists decomposition from light or air for months, making storage and handling less stressful.
Technical Specifications & Labeling
Labeling details do not aim to confuse anyone. Chemical suppliers usually assign CAS number 39486-51-0. Certificates of analysis guarantee at least 98% purity for research-grade material. Common safety markings reflect its basic nature and the fluoride content in the anion. You’ll also see GHS pictograms warning about skin and eye irritation, with risk phrases alerting users to possible toxicity in case of ingestion or prolonged skin contact. Container labels spell out the need for cool, dry storage and include a reminder to keep TMG tetrafluoroborate tightly capped, away from acids, moisture, and food storage.
Preparation Method
Simple, reliable synthesis has helped TMG tetrafluoroborate gain traction. Manufacturers usually start with tetramethylguanidine—prepared by methylating guanidine—or buy it commercially. Anhydrous hydrogen tetrafluoroborate reacts directly with TMG in a dry, inert solvent, generally acetonitrile. This approach knocks down most water and guarantees clean crystals. The product falls out, gets washed with cold ether, and then air-dried until it’s free-flowing and ready for packaging. Scale-up for industrial use demands careful handling of hydrogen fluoride sources, but most routine academic or small-scale syntheses happen without major hang-ups.
Chemical Reactions & Modifications
The core role of this compound matches the history of TMG itself—serving as a powerful, bench-stable base. TMG tetrafluoroborate frequently pops up in alkylation, acylation, or cyclization reactions, especially when researchers worry about moisture or competing nucleophiles. On rare occasions, the BF4- anion swaps out for others during metathesis, opening up new reaction pathways or ionic liquid formulations. The salt’s utility in generating reactive intermediates, particularly those that can decompose in the presence of strong aqueous base or acid, keeps it in the toolkits of synthetic chemists. It also finds use in catalyzing formation of heterocycles, activating alkyl halides, or serving as a base in C–H activation reactions.
Synonyms & Product Names
Tetramethylguanidine tetrafluoroborate appears under a handful of names. Laboratories sometimes call it TMG-BF4 or N,N,N’,N’-Tetramethylguanidine tetrafluoroborate. Catalogs may turn up the less common trimethylaminoguanidine tetrafluoroborate, but these refer to the same salt. Keeping tabs on synonyms avoids confusion during ordering or data searching, as even major chemical suppliers can vary in how they describe the compound. Regardless of the name, experienced chemists recognize it by structure and behavior more than by label.
Safety & Operational Standards
Handling TMG tetrafluoroborate does not raise major red flags compared to many strong organic bases. Still, its strong basicity and the presence of tetrafluoroborate mean gloves, lab coats, and splash goggles are non-negotiable. Direct skin contact stings and eyes take a serious beating from even minor splashes, so proper personal protective equipment remains essential. The salt burns if exposed to strong heat and can release hazardous fumes, including fluoride-containing vapors, if broken down or incinerated. Researchers take pains to keep it away from mineral acids, which might liberate toxic BF3 gas. Standard protocols use fume hoods for large-scale transfers, and fire extinguishers are kept nearby in the event of accidental overheating. Local waste guidelines direct proper disposal, treating contaminated materials as hazardous, especially because fluorinated compounds often resist easy breakdown in municipal treatment plants.
Application Area
Organic chemical synthesis stands out as the main application zone for TMG tetrafluoroborate. Chemists working in academic research lean heavily on it for making complex molecules, particularly when sensitive or multi-step procedures call for a dry, reliable base. Pharmaceuticals development, dye synthesis, and materials science incorporate it in early-stage processes where side reactions must be controlled. In the past decade, electrochemistry and green chemistry movements have begun exploring this salt for use in ionic liquids and advanced catalysis regimes. Enzyme mimetics and organocatalysis—fields that push the boundaries of mild, selective chemistry—often cite TMG salts as key ingredients for screening new transformations. Patents over the years build on its ability to both deliver strong basicity and stay easy to handle compared to older inorganic bases.
Research & Development
Development teams and academic labs draw up plans for improving both the synthesis and downstream uses of TMG tetrafluoroborate. Efforts to prepare this salt from renewable precursors tie into the broader shift toward sustainable chemistry. Modifications of either the guanidine base or the tetrafluoroborate anion target enhanced solubility or boost reaction rates for emerging transformations. Research dives into mechanistic studies, mapping out how this compound influences reaction pathways or intermediates, especially in organocatalytic cycles. Analytical chemists fine-tune methods for rapid detection, trying to catch trace impurities that can alter reaction outcomes and optimizing conditions for safe scale-up. Even the packaging and supply chain draw attention, as researchers request forms that stay stable without extravagant refrigeration, supporting field work or operations in developing regions.
Toxicity Research
Not every chemist likes to talk about toxicity until it lands in their inbox, but exploring the risks tied to TMG tetrafluoroborate has saved more than a few headaches. Acute oral toxicity sits in the low-to-moderate range for rodents, according to recent studies. Dermal and ocular contact show quicker, more severe effects, so protecting exposed skin stays front-of-mind. Long-term studies remain somewhat limited, with available data hinting at possible kidney and liver effects in high-dose animal tests. Decomposition under fire or prolonged acid exposure poses the biggest hazards, as liberation of boron- or fluoride-based species can bring systemic toxicity or corrosive burns. Suppliers recommend annual safety training for staff frequently working with any tetrafluoroborate, and most modern labs keep emergency eyewash stations and spill kits within arm’s reach.
Future Prospects
Tetramethylguanidine tetrafluoroborate stands at a crossroads in synthesis, green chemistry, and industrial processes. The drive to cut down hazardous waste and streamline organic reactions places it on shortlists for next-generation catalysts and ionic liquids, with researchers focusing on reducing residual fluoride contamination in end products. As new carbon–carbon and carbon–heteroatom bond-forming reactions demand ever-narrower selectivity, the clean, strong basicity of TMG tetrafluoroborate holds promise for creative breakthroughs. Advances in toxicity control, supply chain sustainability, and refined analytical monitoring all play into the vision of widespread, responsible use in the next decade. Scientists remain keen to see how tweaks to the guanidine core or the anion partners could unlock new solution properties, giving the old workhorse fresh legs in modern labs.
Inside the Lab: Practical Uses and Reactions
Chemists have a toolbox in the lab, and some tools work better than others for specific tasks. Tetramethylguanidine tetrafluoroborate, known among researchers as TMG-BF4, fits a specific spot in that kit. In my own synthetic work, choosing the right base made or broke the experiment. TMG-BF4 stands out because it offers the strong basicity of tetramethylguanidine paired with the stability and easy handling of a crystalline salt.
This compound shows up in organic synthesis, where conditions often challenge stability and safety. Handling pure tetramethylguanidine takes care because it’s a liquid that fumes and irritates skin. By pairing it with tetrafluoroborate, you end up with a solid that’s much friendlier to store, weigh, and use. The result: precision goes up, spillage goes down. In the real world, nobody wants a runaway reaction just because a base drifted across the bench top. The crystalline form of TMG-BF4 helps sidestep that headache.
Why TMG-BF4 Has a Following in Modern Chemistry
The story doesn’t stop with convenience. In the lab, strong organic bases help form carbon-nitrogen, carbon-carbon, and other bonds that lie at the heart of materials science, pharmaceuticals, and agricultural chemistry. Over the past decade, reports and patents keep referencing TMG-BF4 because it unlocks selectivity and power in tough reactions. For example, making heterocycles—the building blocks for many drugs—often needs a push that weaker bases can’t give. TMG-BF4 steps in, refuses to get distracted by water or atmospheric CO2, and keeps the reaction sharp on its target. That reliability matters when each synthesis costs time and money.
More chemists now try to reduce their environmental impact. Compared to mineral bases like sodium hydroxide—caustic, aggressive, and fussy about incompatible functional groups—TMG-BF4 lets sensitive molecules survive reaction conditions intact. That opens doors to more sustainable reaction routes, with higher yields and fewer byproducts heading to waste. I’ve seen green chemistry teams choose TMG-BF4 for this reason alone. Less mess, more efficiency, happier regulators.
Risks, Hazards, and Responsible Handling
Solid bases always draw attention for use in illicit drug manufacture, so responsible sourcing and recordkeeping matter for institutions and suppliers. The point isn’t to stoke alarm—just a reminder that powerful chemicals require oversight. In my research group, adoption of TMG-BF4 didn’t just speed up troubleshooting; it also brought new lab protocols. We kept it under lock, did thorough risk assessments, and trained every student and staff member who opened the container. Safety and accountability go hand-in-hand.
Manufacturers invest resources in making TMG-BF4 available at high purity and consistent quality. Contamination or degradation in sensitive chemical reagents leads to faulty experiments or failed scale-up in industry. It pays to work with trusted suppliers and verify batch certificates before launching into multi-step syntheses. Reliable sourcing supports reproducibility and safety all the way from the benchtop to pilot plant.
Looking Ahead: Innovation and Opportunity
Interest in TMG-BF4 ties into the broader search for efficient, greener chemistry. Scientists keep tinkering with new applications—from streamlining medicinal chemistry projects to enabling cleaner energy tech via advanced materials. Staying updated on safe practices, regulatory snapshots, and the latest research means chemists can keep this compound working for progress, not against it.
Why Safety Matters in the Lab
A lab can feel like a safe space for those who understand their materials. That sense of security comes from a mix of practice, respect for the tools, and knowing what you’re working with. I remember early days in academic research, where a compound could seem harmless because it had never caused trouble. Comfort leads to shortcuts, which almost always end badly. Tetramethylguanidine tetrafluoroborate isn’t as famous as some chemicals, but it’s a good example of why every substance deserves a closer look.
The Risks of Tetramethylguanidine Tetrafluoroborate
This compound, used in organic chemistry as a base, doesn’t give off fumes or smells that set off alarm bells. But safety comes down to more than what your senses tell you. The core component, tetramethylguanidine, draws concern among chemists for its ability to irritate skin, eyes, and even the respiratory tract. Anyone who has experienced a chemical in the eye knows how agonizing it can be; irritation means real discomfort and sometimes long-term harm without swift response.
If swallowed, the compound’s basic nature threatens to corrode tissue. A splash on the skin may cause burning or redness. Gloves, goggles, and working in a fume hood aren’t up for debate with chemicals like this. The salt—tetrafluoroborate—brings its own mess of concerns. Research shows some tetrafluoroborate salts break down to release hydrofluoric acid (HF), a chemical notorious in the lab world. Even a small exposure to HF can end up far more severe than a first glance suggests, since the acid attacks bones and disrupts calcium levels, leading to deep tissue damage. Not every exposure triggers all these issues, but under the right circumstances, trouble follows.
Chronic Effects and Environmental Impact
Working with dangerous chemicals may not always show immediate harm. After years in various chemistry labs, I’ve met colleagues dealing with chronic skin complaints or nerve symptoms, not always knowing the root cause. Literature on tetramethylguanidine compounds warns about long-term risks through inhalation or repeated skin contact. Risk doesn’t mean guaranteed harm but underlines the value of treating this compound with care. Chronic health problems often sneak up—better practices early keep you in better shape later.
Disposal brings another challenge. The fluoride ions from tetrafluoroborate, once released, persist in the environment. Aquatic life feels the hit first, but these ions don’t just disappear. Only trained staff should handle waste from fluoride-containing compounds. That’s not just bureaucratic red tape—it’s local water and food supply at stake. I’ve seen how just a few bad practices from one lab carry downstream for years.
Sensible Steps Forward
Every compound, even those without warnings on the bottle, deserves respect. Suppliers usually provide a safety data sheet (SDS), sometimes dry reading, but packed with the best available information. No chemist fails to overlook a hazard, but systems and culture catch those mistakes. Written protocols, regular inventory reviews, and real, hands-on training take time but save headaches later. Whether a first-year college student or an industry veteran, nobody has immunity from accidents. Wearing the right gear, labeling everything, and storing chemicals properly protects not just the user but everyone sharing that space.
Tetramethylguanidine tetrafluoroborate demands good habits. That’s the bottom line. Respect the chemical and it remains a tool—not a threat.
Understanding the Basics
Chemistry textbooks often pack in terms and formulas that look intimidating at first glance. Tetramethylguanidine tetrafluoroborate doesn’t roll off the tongue, but break it down and it’s not so mysterious. The chemical formula is C5H13N3·BF4. It’s built from a core of tetramethylguanidine—an organic base—paired up with the BF4− anion. The tetrafluoroborate brings stability and counterbalances the positive charge from the tetramethylguanidinium cation.
Why Should Anyone Care?
Working in a research lab a few years ago, I ran across tetramethylguanidine tetrafluoroborate during a synthesis that required a stable, non-nucleophilic base. Plenty of labs look for alternatives to traditional bases that spark side reactions or bring in contamination. This salt offers an answer by keeping reactivity in the places you want and not adding more headaches somewhere else.
Stability matters when chemists design sensitive syntheses. The tetrafluoroborate anion doesn’t react easily with most of the other chemicals floating around in the flask, which helps avoid nasty surprises. Instead of dealing with breakdown products or rearrangements, researchers can focus on the main reaction. This translates to better yields and fewer failed runs. That reliability saves both time and money, stretching already tight research budgets further.
Structure: Simple, Yet Smart
Look closely at the structure. The tetramethylguanidinium ion centers around a guanidine skeleton, surrounded by four methyl groups on the nitrogens, creating a sort of chemical umbrella. Picture a compact central core, with the positive charge distributed across nitrogens, each shielded by a methyl group. The tetrafluoroborate part itself forms a nearly perfect tetrahedral shape, with four fluorine atoms sitting at the corners, attached to a central boron atom. The salt falls into a class of ionic compounds—a positive cation, and a negative anion, locked together by electrostatic attraction. This pairing means you don’t get runaway reactions; you get control.
Safety and Practical Considerations
In the hands-on world, safety counts for more than theoretical yield. Tetramethylguanidine on its own acts as a strong base and can mess with skin and eyes if you treat it carelessly. The tetrafluoroborate salt dulls some of that behavior by forming a stable compound that’s easier to handle, measure, and store. Even so, gloves and eye protection aren’t optional. The tetrafluoroborate component demands respect, too—fluorinated compounds can produce toxic byproducts if they end up in high heat or strong acids, so keeping things cool and contained remains a must.
Looking Forward: Research and Solutions
Chemists grapple with finding cleaner and safer routes to synthesize medicines, materials, and everyday products. Tetramethylguanidine tetrafluoroborate steps in as a reliable workhorse, capable of making some tricky reactions much more manageable. Staying alert to safer alternatives and always double-checking disposal methods helps keep workplaces and the environment secure. Research never stands still, and as more is learned about this salt, new uses likely emerge, pushing chemistry ahead one careful experiment at a time.
Why Proper Storage Matters For Chemical Safety
Many folks do not give storage a second thought, especially with complex names like tetramethylguanidine tetrafluoroborate. This chemical sounds esoteric, but those who handle chemicals in labs and manufacturing deal with similar compounds all the time. Lax storage puts health, productivity, and research at risk. Nasty accidents do not warn you before they happen. I have seen storage rooms turn into safety hazards because somebody got too comfortable.
Handling Chemicals with Respect
Tetramethylguanidine tetrafluoroborate affects more than just glassware. This salt may not catch fire like organic solvents, but it packs a punch if moisture gets in. Water can start harmful reactions, breaking down the compound or making it corrosive to containers, benches, and even skin. I learned early that an airtight vessel beats any paper warning taped to a bottle. A bottle with a dried-out cap or loose threads can make the best-stocked cabinet dangerous.
Storing this chemical in a tightly-sealed container, preferably glass or high-quality plastic, helps avoid accidental contact with humidity. I remember my supervisor’s insistence on labeling the date a bottle arrived and checking for any damage, just to make sure nothing degraded over time. Most accidents I’ve seen started with “It’s probably fine” — only for someone’s day to end in a decontamination shower.
Choosing the Right Spot
A dry, cool, ventilated place keeps most reactive salts out of trouble. I always kept similar chemicals away from sinks, water lines, or window ledges, which seem harmless until something leaks or condensation finds a route. Storing on a middle shelf — not the top or bottom — stops clumsy hands from causing a fall or bottle breakage.
Chemicals with tetrafluoroborate make acids if they see moisture. Contact with skin or eyes results in burning and hospital trips. Separate your storage by family: put basic compounds far from acids and bases to avoid accidental mix-ups. Even the cleanest labs I’ve worked in stock different compounds just inches apart, so clear danger signs and detailed logs aren’t optional.
Using Facts, Not Hunches
The Safety Data Sheet for tetramethylguanidine tetrafluoroborate recommends dry, waterproof storage and strong labeling. Large chemical suppliers and research organizations echo these facts, and ignoring them is asking for an emergency. I have trusted guidance from experienced safety officers more than any online summary or hearsay.
Reacting quickly when a container gets damaged or leaks saves time and cash in the long run. In one university lab I worked in, students learned fast to report cracks or sticky caps, so maintenance could replace containers before issues multiplied. No one wanted another evacuation drill after fumes ruined a week’s experiments.
Practical Steps Everyone Should Take
- Find a cool, dry area with limited access
- Keep containers tightly closed and upright
- Use clear, chemical-resistant labels
- Separate from incompatible materials
- Review condition of bottles at least monthly
- Keep emergency spill kits close — not stashed in a distant corner
Safety takes persistence and respect for the risks. Good habits in chemical storage shield people and projects alike. Every seasoned lab worker picks up these habits for a reason — and often, it comes from firsthand stories about what happens if you neglect them.
Packaging Choices Come Down to Utility and Safety
Anyone working with specialty chemicals like tetramethylguanidine tetrafluoroborate finds that simple questions about packaging aren’t so simple after all. Safe handling means a lot more than just picking a convenient bottle off a shelf. Most lab chemicals, especially those with a niche market and distinct hazard profile, won’t show up in a rainbow of packaging varieties like breakfast cereals. Instead, packaging stems from balancing safety with the right amount for the job.
Lab researchers talk to each other about suppliers as much as they talk shop about research results. Word spreads fast if a supplier packages this material in flimsy containers, or if the bottle always matches the order’s needs. In most catalogs and MSDS sheets, tetramethylguanidine tetrafluoroborate typically comes in small-volume containers—ranging from just a few grams up to 100 grams. Bulk isn’t the name of the game for this one. Large-scale users often set up special contracts, but those options rarely appear in general sales channels.
Standard Sizes: Options on the Ground
Browsing suppliers such as Sigma-Aldrich, TCI, or Alfa Aesar, it’s clear that glass bottles sealed in protective packaging dominate the scene. Most offerings land in the zone of 5 grams, 25 grams, 50 grams, and at the stretch, 100 grams. Smaller bottles give labs more control and minimize losses if spills or contamination happen. Larger packs, though available, tend to cost less per gram, suiting bigger projects, yet rarely show up on public-facing webstores due to storage concerns and the specialized nature of the compound.
Shipping remains a big part of why packaging sizes matter. Couriers have no interest in loose powders bouncing around trucks, and neither do research managers. Regulatory rules influence sizes as well. For example, the United States Department of Transportation and international equivalents restrict the maximum net weight for hazardous chemicals shipped by air or ground. Most chemical suppliers won’t budge far from those thresholds.
Why Small Counts: User Experience and Risk Management
In practice, chemical research involves trial runs before scaling up. Nobody wants to tie up budgets in large packs that expire before they get used. Storage stability enters the frame; tetramethylguanidine tetrafluoroborate often works best kept in tight-sealing bottles, away from moisture and oxidizers. Distributors choose amber-glass or high-density polyethylene containers with PTFE-lined caps, prioritizing chemical compatibility over flashy branding.
Any time a new project calls for more than the standard amounts, the supply chain turns into a negotiation. Bulk orders or custom drums require direct talks with the supplier and, many times, extra paperwork for hazardous transport. The storage risk piles up fast, both for suppliers and labs. That’s why most research teams stick to the smallest effective size, order only what the project can consume, and opt for suppliers with reliable safety records.
Looking Ahead: Improving Access Without Sacrificing Safety
The big challenge in packaging specialty chemicals like tetramethylguanidine tetrafluoroborate lies in supporting both laboratory flexibility and responsible chemical management. Clear, standardized labeling, and concise product data from suppliers take some guesswork out of procurement. Supply partners who offer transparent batch information, safety data, and prompt communication do more than just sell a bottle—they help create a safer work environment.
Experience has shown me that reaching out to suppliers with specific needs often uncovers tailored solutions, sometimes outside of the visible catalog. For green-chemistry projects or scale-up campaigns, direct supplier contact gives more options than a quick web order. Ultimately, sticking with right-sized bottles, stored with care, keeps both projects and people out of harm’s way.