Tetramethylguanidine Trifluoromethanesulfonate: In-Depth Commentary
Historical Development of Tetramethylguanidine Trifluoromethanesulfonate
Chemistry never stands still. Decades ago, organic chemists worked with guanidine derivatives to overcome persistent limitations in traditional acid-base reactions. Tetramethylguanidine (TMG) itself first made waves by delivering unmatched basicity along with modest steric hindrance, which gave rise to applications across pharmaceuticals and materials synthesis. Only after realizing the value of pairing strong organic bases with specialized counterions did researchers attempt combining TMG with trifluoromethanesulfonic acid. The resulting salt, tetramethylguanidine trifluoromethanesulfonate (TMG·TfO), benefits from both the nucleophilic boost of guanidine and the stable, non-coordinating nature of triflate. This hybrid took root in the late 20th century as the smart answer for catalysis and selective transformations, especially where older salts delivered cluttered outcomes or left behind problematic residues. In my experience, few compounds highlight chemical creativity quite like this one does, becoming a linchpin in ambitious synthetic projects.
Product Overview and Synonyms
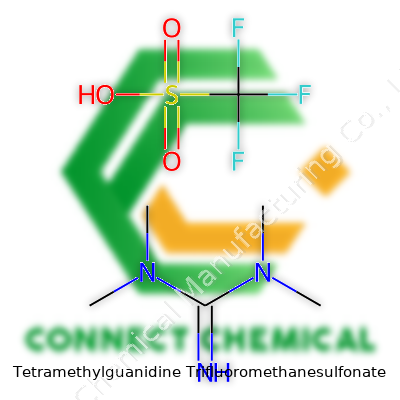
By itself, TMG triflate stands out among specialty reagents. The full IUPAC name tells the tale: 1,1,3,3-Tetramethylguanidine trifluoromethanesulfonate. Suppliers also label it as TMG·TfO or simply tetramethylguanidine triflate. No matter the naming, the attention lies in its capacity to function as a strong, stable, and dry source of guanidinium with a reliable counterion. Chemistry teams hunting for solid, isolable alternatives to older bases pick TMG·TfO to avoid the unpredictable swings sometimes seen with aqueous or gaseous bases. Over the years, chemical catalogs have broadened, but few compounds manage that balance of potency and manageability for lab-scale and industrial use alike.
Physical and Chemical Properties
In the lab, TMG triflate comes as a white to off-white crystalline solid. Chemists appreciate its pronounced solubility in polar aprotic solvents such as acetonitrile and dimethylformamide, which fits standard synthetic workflows. Structurally, this salt stays stable under typical storage conditions if kept away from moisture. The triflate anion, with its three fluorines, imparts not just stability but also thermal endurance, often carrying operations safely beyond 100°C without major decomposition. Analytical measurements confirm a melting point generally above 150°C and robust purity profiles in commercial preparations. Chemical reactivity draws from both the strong basicity of the guanidine and the weakly coordinating nature of the triflate, putting powerful tools into chemists' hands while minimizing secondary interactions that can throw reactions off track.
Technical Specifications & Labeling Practices
Chemical suppliers tend to offer TMG triflate in purity ranges above 98%, with certificates of analysis to back each batch. Labels detail molecular formula (C5H13N3·CF3SO3H), molecular weight (307.31 g/mol), and CAS number for transparency. Precautionary phrases and hazard pictograms signal the need for protective equipment, especially eye and skin protection. Product documentation generally brings up storage recommendations—cool, dry locations away from acids or bases—since long exposure to air and humidity can degrade salt integrity or alter its solubility. Reliable traceability, down to exact lot numbers and synthetic methodology, helps labs meet increasing regulatory scrutiny related to research reproducibility.
Preparation Method
Lab-scale routes to TMG triflate are practical and safe, building trust through reproducible yield and purity. The synthesis starts with equimolar mixing of tetramethylguanidine with trifluoromethanesulfonic acid, typically in an anhydrous organic solvent. Stirring under cool conditions ensures controlled release of heat while forming the crystalline salt. Once complete, the mixture gets filtered and dried under vacuum, producing TMG triflate with only residual solvent to remove. As I have seen, bench chemists appreciate this straightforward approach since it requires only standard glassware and minimal waste disposal. Advanced tech routes now support multi-kilogram batches for industrial settings, further boosting its reputation as an accessible, scalable specialty reagent.
Chemical Reactions & Modifications
TMG triflate rarely sits idle on a shelf. Its main draw lies in enhancing nucleophilicity and selectivity where older ammonium or guanidinium salts fall short. In peptide coupling, for instance, it activates carboxylic acids without complicating side reactions, outperforming many traditional acid-base pairs. In heterocycle construction and natural product total synthesis, TMG·TfO can push challenging cyclizations through by ensuring a dry, base-rich but non-nucleophilic environment. Chemists also use the salt as a modulator in organocatalysis, improving catalyst turnover and operational simplicity. In research collaborations, I’ve watched TMG·TfO unlock transformations previously thought out of reach, simply by taming counterion reactivity or making tough intermediates tractable. Recent years have seen tweaks to the parent salt—adding substituents to the guanidine core or swapping solvent systems—to push selectivity and throughput even further.
Safety & Operational Standards
TMG triflate expects vigilance from operators. Direct skin or eye contact triggers irritation, so full-length gloves and splash-proof goggles are best practice. While not explosively hazardous, its basicity and the presence of the triflate group mean that spills can linger or taint other reagents if not cleaned up thoroughly. Storage protocols stem from its moisture sensitivity—tightly capped bottles and desiccated cabinets give best shelf life, especially in climates prone to summer humidity. MSDS documents spell out exposure routes, safe disposal protocols, and first aid measures, echoing lessons from past workplace mishaps. Industrial users now face tighter workplace air monitoring and environmental discharge constraints, a direct result of increased chemical regulation across North America, Europe, and Asia.
Application Area
Synthetic organic chemistry drives the lion’s share of demand for TMG triflate, especially where high purity and strong, yet controllable, basicity matter. Drug discovery teams have turned to this salt for peptide assembly and small-molecule functionalization, shaving hours from reaction times and reducing unwanted side-product formation. Polymer researchers use it to drive initiator-free or metal-free ring-opening polymerizations, keeping catalysts out of the final material. Recent forays include lithium battery research, where TMG·TfO’s strong yet non-interfering base properties aid electrolyte design. Custom material developers also scale up the salt for films and coatings needing precise cross-linking or modular architectures. In my own circle, colleagues in green chemistry point to TMG triflate as an ally for lowering solvent consumption and reaction waste thanks to its operational efficiency.
Research & Development Efforts
University labs and commercial R&D centers keep testing the limits of what TMG triflate can achieve. Ongoing projects aim to widen compatible substrate scopes and reaction conditions, targeting milder temperatures, broader pH ranges, and fewer purification steps. Next-generation analogs, built on TMG·TfO’s template, focus on supercharging selectivity for value-added fluorinated compounds in agrochemicals and pharmaceuticals. Collaborations between academic and industrial partners dig into process optimization, scaling fine-tuned TMG·TfO reactions for lower cost and greener profiles. Literature surveys show a rising tide of patent filings, reflecting market confidence in this reagent as a building block for complex molecules and sustainable syntheses.
Toxicity Research
Long-term safety research around TMG triflate builds on historical data from guanidines and triflate salts. Acute exposure mainly triggers local irritation, so the risk profile stays moderate with basic precautions. Chronic toxicity studies in mammals show that similar compounds rarely accumulate in tissues due to rapid elimination, though concerns around fluorinated metabolites urge continued vigilance. Environmental impact raises more questions, as triflate residues resist natural degradation and may persist in wastewater. Analytical teams have recently developed new soil and water testing methods to flag contamination early. In laboratory or plant settings, strict adherence to waste treatment standards—especially neutralization and downstream filtration—reduces accidental discharge risks, contributing to safer workspaces and cleaner water supplies.
Future Prospects
With pressures mounting for efficient, reliable reagents, TMG triflate shows real staying power. As pharmaceutical, material, and energy sectors all crave versatile, safe, and scalable building blocks, this salt finds fresh outlets and eager adopters. The next era will focus on greener process design, better recyclability, and simplified downstream cleanup. Ongoing innovation is already reshaping how TMG triflate fits into continuous-flow chemistry, automated synthesis, and AI-aided reaction planning. Tackling the environmental footprint of triflate and its derivatives could lead to breakthroughs in design, regulation, and safe handling. For those of us who value progress at the intersection of lab precision and industrial scalability, compounds like TMG triflate point the way forward in both research and application.
The Real Workhorse Behind Complex Syntheses
Walking into any synthetic chemistry lab, glassware glistens on benches and small vials filled with mysterious powders line the shelves. Researchers reach for reagents that push reactions where they simply won’t go by themselves. Tetramethylguanidine trifluoromethanesulfonate stands out among these helpers. Known for its long name and hefty punch, this compound’s main job comes down to activating other chemicals so new bonds can form.
I remember those long afternoons in the lab during my graduate days. Somebody always had to hunt down the reagent that could coax stubborn molecules into the right shape or get complex machinery running. Tetramethylguanidine trifluoromethanesulfonate usually came up during talks about designing pharmaceuticals or fine chemicals. Its unique acid-base properties mean it helps nudge along reactions by accepting or donating protons. This isn’t just theory; without this chemical, some life-saving drugs would simply take much longer to synthesize, or the yield would drop off a cliff.
Shaping the Future of Medicine and Materials
Pharmaceutical companies chase efficiency. It’s a game of time and purity. Tetramethylguanidine trifluoromethanesulfonate steps in to help form carbon-nitrogen and carbon-oxygen bonds, two connection types needed for complex drug molecules. Studies published in journals like Organic Letters and Angewandte Chemie show just how vital this reagent has become for making nitrogen-containing rings and other tricky architectures. If the old approach took twelve steps, chemists might drop a couple simply by swapping in this reagent.
The impact isn’t limited to medicine. Materials science borrows this reactive powerhouse for designing polymers and high-performance materials. Take the creation of catalysts—these engine parts of chemistry need selective, clean preparation. Using this reagent, scientists achieve higher yields and cleaner endpoints, according to research from institutions such as MIT and University College London.
Safety and Ethical Concerns
Any chemist with experience knows a potent reagent brings its own risks. Tetramethylguanidine trifluoromethanesulfonate, with its strong acid properties and reactivity, requires careful handling. Exposure can irritate skin and eyes, and spills in the lab mean emergency protocols kick in fast. The safety data sheets (from Sigma-Aldrich and other suppliers) urge proper gloves, goggles, and fume hoods. As the science grows, companies must train staff and uphold the highest safety standards.
Costs and Supply Challenges
Not every lab has shelves lined with this compound, mostly due to cost and procurement limits. International supply chains sometimes slow down delivery. Anyone involved in scale-up knows that chemistry on paper runs into real-world supply issues, especially for specialty reagents. The push for greener alternatives drives researchers to look for less hazardous, more available chemicals. Until then, care and planning keep projects on track.
Paving a Responsible Path Forward
Tetramethylguanidine trifluoromethanesulfonate has earned its spot on the chemist’s bench by enabling faster, higher-yielding reactions. Better training and strict safety culture matter as much as its molecular power. With medicine and new technology on the line, the right use of this reagent helps save time, cut waste, and edge science forward.
Understanding Why This Chemical Demands Respect
Tetramethylguanidine trifluoromethanesulfonate tends to come with an intimidating name, and it backs that up with some real hazards. This chemical often gets used in advanced synthesis labs. I’ve seen both seasoned chemists and interns make mistakes if they skip basic safety routines, so this isn’t just “lab manual advice.” Anyone handling it regularly knows spills, splashes, or even unseen vapors have consequences—skin burns, serious eye injuries, or respiratory problems. Given the triflate anion and the notoriously aggressive guanidine base, this stuff doesn’t do anyone a favor when safety gets overlooked.
Simple Gear Makes a Big Difference
Never underestimate a sturdy pair of nitrile gloves. I’ve seen too many folks rely on simple latex—nitrile stands up much better. Lab coats, goggles that actually fit, long pants, and closed shoes stay non-negotiable. For bigger batches or transfers, devote the time to finding a good face shield. PPE feels like one extra step, but getting a chemical splash on your skin will convince anyone to double-check their gear after just one bad day.
The Power of Good Airflow
All the PPE in the world can’t save you from fumes if a reaction goes sideways. Most incidents I’ve witnessed happened in poorly ventilated spaces. Work in a chemical fume hood, keep the sash low, and confirm the ventilation works before setting out any bottles or glassware. If you sense a sharp ammonia-like smell while working, it’s not a sign to tough it out; that’s your cue to step away and let the airflow clear it.
Smart Storage and Prep
This chemical does best in a cool, dry place, away from acids and oxidizers. Separate secondary containment baskets cut down on cross-contamination risk. I’ve seen acids and streamlining solvents get stored together for convenience, but one mix-up can cost someone their eyesight or lungs. Use clear, well-labeled containers and record every time a new batch gets opened or dispensed.
Spill Response Isn’t Just Theory
Fires and spills rarely stick to predictable times. I’ve been in the middle of a reaction when a little moisture in a flask started breaking things down fast. Always keep spill kits close with absorbent pads, neutralizers, and disposable scrapers. If tetramethylguanidine trifluoromethanesulfonate gets loose, evacuate that spot and clean from the outside in. Tossing regular paper towels only spreads the mess and can start dangerous breakdown reactions.
Immediate Response Protects Everyone
Inhalation exposure or skin contact? Head straight for the eyewash and safety shower. Don’t wait, don’t try to “tough it out.” Report exposures right away—delaying reporting can lead to burns worsening or missed medical care. I’ve seen folks shrug off a splash, only to discover blisters an hour later. Nobody should underestimate how quickly this chemical can do damage.
Building A Safer Lab Culture
Every smart safety protocol reflects a few near misses and hard-won lessons. Keep regular training sessions. Share close-call stories. Chemicals with this much reactivity invite problems if you let your guard down for even a minute. Encourage folks in the lab to ask about protocols before starting a new task or scaling up. The stakes are always personal—protect your hands, your lungs, your team’s peace of mind. Safety starts with respect and real, lived caution.
Why Storage Matters for Chemical Safety
Walking into a lab, bottles with long names and stranger uses fill shelves. Among them, Tetramethylguanidine Trifluoromethanesulfonate can sound intimidating, but it’s simply a strong organic base partnered with a tough acid. Its power makes it useful—and risky. Old stories of ruined research and lab evacuations come to mind, all tied to careless storage. Bad habits catch up, especially with potent salts like this one.
Looking At Stability and Storage Realities
Chemicals either make or break research, and storing the tricky ones can mean the difference between success and cleanup duty. Tetramethylguanidine Trifluoromethanesulfonate likes its space dry and cool. Let a humid summer sneak in, and suddenly clumpy powder turns to sludge, wrecking its reactivity. Moisture brings more than a sticky mess—unintended reactions kick off, and purity drops. Chemical suppliers stress anhydrous conditions for storage. Watch any experienced chemist, and you’ll see desiccators and tight seals on every sensitive bottle.
Importance of Labeling and Isolation
Crowded shelves breed confusion. One glance at an unlabeled bottle leads to trouble, as I've learned in crowded university storerooms. Every hazardous chemical asks for clear, permanent labels—dates, names, concentrations—especially if it’s been repackaged. Keeping similar containers together may seem neat, but strong bases like Tetramethylguanidine Trifluoromethanesulfonate mix poorly with acids, oxidizers, or anything that might spark surprise. Isolation, even on the shelf, reinforces safety: separate cabinets, secondary containers, and storage logs all help avoid costly mistakes.
Ventilation and Fire Prevention
Sensitive chemicals need smart airflow—fume hoods, dedicated cabinets, or at least rooms that don’t choke on fumes. While Tetramethylguanidine Trifluoromethanesulfonate avoids the limelight of flammability headlines, nobody wants to roll the dice by stacking open jars near heat sources or ignition points. Even non-flammable powders gain risks once they start reacting, so fire suppression tools stay close at hand.
Training Matters More Than Expensive Cabinets
The fanciest safety cabinet won’t save the day if no one understands the rules. Staff training shows up over and over in incident reports—miss a step, skip a label, or forget to check the seal, and the risk leaps up. Seasonal lab rotations and hurried work make it tempting to take shortcuts, but regular reminders and training keep the basics fresh. I’ve watched colleagues swat away disaster thanks to muscle memory trained by monthly briefings.
Simple Steps for Handling Risky Stock
Whenever Tetramethylguanidine Trifluoromethanesulfonate comes off the shelf, basic gear makes a difference: gloves, goggles, and respectful distance from food or drink. Spills don’t wait for official emergencies—regular checks and spill kits close by save more trouble than most realize. Waste disposal looks dull on paper but ignoring the rules fills up hazardous bins fast.
Learning From Experience, Not Regret
Safe storage starts with respect and routine. Tetramethylguanidine Trifluoromethanesulfonate supports big advances in synthesis, but it rewards care. Sharing storage insights beats learning from cleanup duty or the sound of a fire alarm. With a focus on best practices, a dose of healthy skepticism, and solid training, accidents shrink and discoveries flourish.
Getting to Know Tetramethylguanidine Trifluoromethanesulfonate
Years in the lab teach a person to look past wild technical phrases and see the patterns in molecules that end up driving a reaction. Tetramethylguanidine trifluoromethanesulfonate represents a pairing of two well-known ingredients from the chemical toolbox. On one side, there's tetramethylguanidine, a strong organic base with a knack for deprotonation. On the other, trifluoromethanesulfonic acid (triflic acid), known for turning a basic molecule into a salt that can take on new chemical duties. When these come together, they create a compound with a unique blend of strength and stability.
Chemical Formula and Structure
The chemical formula for tetramethylguanidine trifluoromethanesulfonate is C5H13N3·CF3SO3H. In more compact notation, that’s C6H16F3N3O3S. Chemists see this as an organic cation (tetramethylguanidinium, (Me2N)2C=NH2+) balanced by a counter-anion (triflate, CF3SO3−).
Diving into the structure helps make sense of its behavior. The tetramethylguanidine part looks almost like a three-pronged fork. Its formula in structural form:
- Tetramethylguanidine: N,N,N',N'-Tetramethylguanidine, where the guanidine core (HN=C(NMe2)2) gets capped at each nitrogen by methyl groups.
The triflate (trifluoromethanesulfonate) brings a sulfonate group attached to a carbon loaded with three fluorines. Its formula: CF3SO3−. The triflate anion does not tend to interact strongly with other ions except, notably, during powerful solvation in polar solvents.
The Role in Chemistry and Industry
Working with strong bases in the lab, especially ones as punchy as tetramethylguanidine, often opens up a world of reactions. In practice, chemists use the triflate salt to get reliable performance under tough conditions. The salt form remains stable and easier to handle than the raw amine, particularly when dealing with moisture or reactive intermediates.
The value to industry grows out of this stability. Handling pure tetramethylguanidine can get dicey—exposure risks, storage headaches, and volatility all stack up. By switching to the triflate salt, factories and research labs sidestep those issues. Clean reactions, measured doses, and reduced waste show up as the benefits on the ground. For synthetic chemists trying to drive nucleophilic substitutions, or who demand a no-nonsense base in pharmaceutical or agrochemical synthesis, this salt helps keep workflow smooth.
Scaling reactions safely and repeatably demands predictability. With this salt, surprises decrease and consistency rises. Instead of fighting with a runaway exotherm or dealing with stubborn byproducts, researchers spend more time on discovery and less time troubleshooting failed runs.
Looking Forward: Responsible Use and Possible Tweaks
Lab workers and factory operators always weigh the safety and environmental footprint of what they use. Tetramethylguanidine trifluoromethanesulfonate enjoys good shelf stability and reduced volatility, making it less likely to escape into the environment compared to free base forms. That said, the triflate ion brings persistent fluorine content. Responsible waste handling, solvent recovery, and consideration for greener alternatives stay important. Researchers keep an eye out for similar salts using less persistent counter-ions, or for better recycling methods for triflate-containing waste streams.
At the end of the day, the blend of muscle and composure wrapped up in this salt helps both researchers and industry users wield a powerful tool, while still keeping an eye on safety, performance, and environmental responsibility. That makes tetramethylguanidine trifluoromethanesulfonate more than just another reagent on the shelf—it’s a bridge between potent chemistry and better real-world practice.
Real-Life Reactions—Not Just Theory
I’ve handled my fair share of tricky reagents over the years, and Tetramethylguanidine trifluoromethanesulfonate (let’s just call it TMG-OTf) always sparks debate in the lab. Chemists get excited about using strong organic bases with reliable counterions, and TMG-OTf fits the bill. Still, nothing in a bottle tells you everything about how it’s going to play along with the regular crowd of solvents and commonsense reagents. We have to look beyond the label and tax ourselves with some good old-fashioned lab skepticism.
Getting Along with Solvents
Water and TMG-OTf don’t mix. It’s not just a matter of solubility—this compound’s strong basicity means it snaps up protons and can rip apart water, releasing heat and forming puzzles no one wants in a reaction flask. Most polar aprotic solvents—think acetonitrile, DMF, DMSO—handle TMG-OTf fairly well. These solvents don’t offer up any protons, so the base won’t pick a fight. I’ve seen smooth workups with dichloromethane or THF, too, with no visible surprises during scale-ups. That said, alcohols and protic solvents produce headaches; the base strips them of their protons and ruins both itself and the alcohol. More than one chemist has learned this after a ruined column or a mystery side product in their NMR spectra.
Classic Reagents Have Stories to Tell
TMG-OTf’s triflate counterion looks innocent but deserves respect. Triflate doesn’t stick around to soak up trouble, and it won’t get involved in side reactions. Strong nucleophiles like Grignard reagents or alkoxides don’t play nicely with this base, since the guanidine backbone latches onto protons fast and can outpace your main reaction. With acid chlorides, I’ve had to watch for rapid heat evolution and flaming exotherms—TMG-OTf doesn’t tiptoe around reactive acyl compounds. Halides and simple salts stay pretty inert, so no headaches there. Typical functional group checks—esters, amides, nitriles—usually pass; TMG-OTf leaves them alone unless pushed into harsh conditions.
Why It Matters—Safety, Cost, and Waste
Chemistry labs can’t afford surprises when mixing unfamiliar chemicals. A strong base like TMG-OTf can ruin carefully planned syntheses if you ignore how it interacts with your solvents and reagents. Once, I watched a student lose a whole week’s work because a reaction with a protic solvent spiraled into decomposition. Worse, disposal becomes trickier if TMG-OTf reacts with water and gives off heat or toxic by-products. Handling waste with an eye on unintended reactivity keeps everyone safe and protects resources.
Solutions—Knowing and Sharing
Product data sheets and reputable references lay the foundation, but nothing beats double-checking with small-scale tests before launching full syntheses. Open notes in the lab, whether it’s a log on paper or a digital file, save the next person from running into the same trouble. Vendors and academic groups publish plenty of compatibility charts, yet every batch, every experiment, shapes its own reality. A blend of published work, lived experiences, and honest troubleshooting sharpens our choices. For those on the bench, clear communication and caution win every time over assumptions.