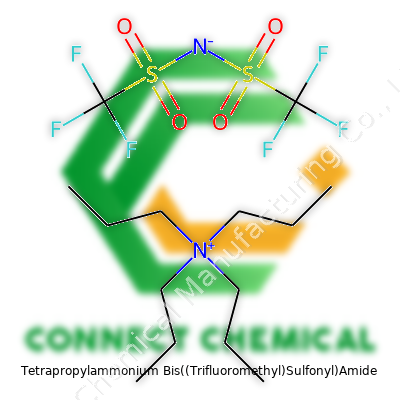
Tetrapropylammonium Bis((Trifluoromethyl)Sulfonyl)Amide: A Complete Commentary
Historical Development
Chemistry doesn’t often make the nightly news, but when you look at the history behind tetrapropylammonium bis((trifluoromethyl)sulfonyl)amide, you find a story of real progress. In the early years of ionic liquids and advanced salts, labs pushed boundaries to go beyond old-school solvents and electrolytes. Industry had its eye on compounds that could offer high stability, low volatility, and new ways to dissolve metals or catalyze reactions. Researchers saw the appeal of perfluorinated sulfonyl anions for their resilience, and the combination with tetrapropylammonium surfaced as a way to further boost utility. Chemists started testing these combinations in the late 1990s, finding they resisted hydrolysis and had unusual solubility patterns. Compared to volatile organic solvents or corrosive inorganic acids, this compound promised cleaner processes and fewer hazards, opening fresh territory for research and production.
Product Overview
Tetrapropylammonium bis((trifluoromethyl)sulfonyl)amide isn’t just another lab curiosity. Laboratories, battery manufacturers, and catalysis groups see in this molecule a modern alternative for creating safer, more efficient reaction media. It’s not prone to catch fire, it doesn’t give off foul fumes under reasonable conditions, and it brings chemical versatility that allows for applications ranging from solvent systems to electrolytes. With the drive for greener labs and sustainable industry, compounds like this move to the front of the shelf.
Physical & Chemical Properties
Look up the compound and you’re met with a clear, crystalline solid or colorless liquid, depending on humidity and temperature. It dissolves in polar solvents such as water, acetone, or dimethyl sulfoxide, offering easy mixing where other salts clump or form messy precipitates. Its melting point hovers around 50–75°C, and it stays stable across a wide temperature range. The bis(trifluoromethyl)sulfonylimide anion stands out for its symmetry and delocalized charge, which encourages high ionic mobility in solution—key for batteries and separations. The molecule also resists degradation by acids, bases, and even strong oxidizers, so it keeps performance up even after extensive cycling.
Technical Specifications & Labeling
Manufacturers often supply this compound with purity higher than 99%, which means impurities rarely interfere with sensitive processes. Typical labeling lists moisture content under 0.5% and notes the CAS number for easy verification. Anyone ordering the substance for regulated uses wants detailed certificates of analysis, including elemental breakdowns and evidence of low heavy metal contamination. Shipping labels warn of mild irritancy and call for gloves and goggles, reflecting good lab sense rather than acute danger.
Preparation Method
Preparation in the lab takes some planning. Chemists combine tetrapropylammonium bromide with lithium bis(trifluoromethyl)sulfonylimide in a water or acetonitrile solution. A double-displacement reaction follows, and filtration removes lithium bromide byproduct. Repeated washing and vacuum drying yields the pure salt, and each lot sees further purification if required for battery or pharmaceutical-grade uses. The method avoids harsh reagents and builds on processes long familiar to trained personnel.
Chemical Reactions & Modifications
The main draw here comes from the anion’s stability and the cation’s moderate size. In practice, this means researchers add the salt to widen the electrochemical window or to study solvent-free reaction pathways. The compound resists nucleophilic attack and remains unchanged in most mild organic transformations. Some teams swap the cation or modify the extent of alkylation to tune solubility, melting point, or conductivity. The symmetrical anion can act as a stabilizing influence in organometallic syntheses, which opens doors for reaction discovery.
Synonyms & Product Names
A single chemical often wears many names. Trade catalogs list it as Tetrapropylammonium bis(trifluoromethanesulfonyl)imide, TPATFSI, or sometimes just “tetrapropylammonium TFSI.” Variations in nomenclature appear across different countries and patents, which challenges ordering but keeps suppliers honest. Keeping track of synonyms is important for cross-referencing literature or sourcing high-purity material.
Safety & Operational Standards
Nobody’s eager to mess with dangerous compounds, and this salt scores high among safer options. You still want gloves, goggles, and good ventilation, especially during weighing or transfer. The compound has no known explosivity or acute inhalation risk, but it can irritate skin and eyes after prolonged contact. Labs comply with REACH, OSHA, and country-specific standards around chemical handling, disposal, and spill response. Clean storage conditions keep cross-contamination low, and proper labeling helps avoid mix-ups—never a laughing matter in any real laboratory.
Application Area
For years, lithium batteries gobbled up new electrolytes, and chemists turned to tetrapropylammonium bis(trifluoromethyl)sulfonyl)amide as a consistent performer. Its high conductivity and broad electrochemical window support both research prototypes and scale-up runs. Beyond batteries, the salt finds use in organic synthesis, where it stabilizes reactive intermediates and acts as a phase transfer catalyst. Some water treatment plants and separation technologies also rely on its ion-pairing abilities to remove heavy metals or boost extraction yields. The push for sustainable chemistry has only grown this pool of applications, and new uses turn up year after year.
Research & Development
Current R&D focuses on knocking down costs and further raising purity. Teams also experiment with analogs, changing alkyl chain lengths or switching to mixed cations. Pilot projects test how subtle tweaks affect ionic mobility and resistance to thermal breakdown. The material sciences crowd especially values collaborations that cross from basic molecular design into real-world devices. This intersection often reveals new routes to value and better serves the shifting energy landscape. Industry and academia share both patents and open literature in ongoing efforts to expand practical knowledge while refining process standards.
Toxicity Research
Safety hasn’t always kept pace with innovation, but recent years brought tighter scrutiny. Animal studies and predictive toxicology models show that the salt has low acute oral and dermal toxicity. Environmental persistence remains under the microscope due to the fluorinated groups in the anion. Scientists measure aquatic toxicity and decomposition pathways, trying to close safety data gaps before expanded industrial use. Fact-based risk assessment depends on transparent reporting and regulatory review, so ongoing research fills in gaps and keeps users informed.
Future Prospects
With electronics advancing, more electric vehicles on the road, and demand for clean solvents spreading fast, tetrapropylammonium bis(trifluoromethyl)sulfonyl)amide faces an open runway. Labs and companies seek lighter, safer, and longer-lasting materials for batteries and renewable energy storage. If supply chains adjust to produce higher volumes with careful waste management, this salt could support greener manufacturing. Technical hurdles will fall with industry investment in process optimization. From my experience in materials research, every gain in purity, stability, or process simplicity delivers downstream benefits, setting the stage for new products that weren’t practical a decade ago. Collaboration and real-world data will keep the compound valuable, as long as research continues to answer environmental and safety questions.
Understanding the Real-World Uses
Staring at a name like Tetrapropylammonium Bis((Trifluoromethyl)Sulfonyl)Amide, most folks outside a chemistry lab might feel lost. In a practical sense, this compound forms the backbone of a new class of materials in advanced chemistry circles. I first crossed paths with it during research into ionic liquids. Scientists don’t pick these materials because of fancy names—they look for stability, solubility, and unique properties.
Powering Modern Ionic Liquids
Lab workers and industrial chemists often talk about ionic liquids these days. They like that these liquids rarely evaporate and can be tuned to slip into all sorts of applications. Tetrapropylammonium Bis((Trifluoromethyl)Sulfonyl)Amide often appears at the heart of these mixtures, especially where high conductivity matters and regular salts get in the way. Having helped prep ionic liquids for battery tests, I’ve seen this material push the edge on what electrolytes can do, lasting longer and handling bigger voltages than most classic choices.
Transforming Electrochemistry
In labs working on next-gen batteries or devices like fuel cells, this chemical stands out. It gives researchers a shot at membranes and solvents that won’t break down or catch fire at high temperatures. That's a big deal on the shop floor and in clean energy projects. It helps run tests that tell us if cheap, safe batteries might soon store solar power on entire city grids. Solid results from universities and companies back this up, showing that batteries based on these ionic liquids often outlast older systems.
Greener Chemistry Without Trade-offs
Most who have worked with harsh or pungent solvents know the hazards and trash problems that come with them. Tetrapropylammonium Bis((Trifluoromethyl)Sulfonyl)Amide-based solutions step around many of those problems. They don’t vaporize easily. They keep their cool under heat, cutting down fire risk. Waste management gets easier, and air in the lab feels a lot safer. Specific reports from the American Chemical Society have tracked the shift in labs choosing these safer solvents for everything from dissolving rare earth metals to building new catalysts.
Building a Basis for Innovation
There’s more here than just batteries. Electroplating, deep cleaning, and even some pharmaceutical manufacturing rely on precise solvents and electrolytes. Here, Tetrapropylammonium Bis((Trifluoromethyl)Sulfonyl)Amide-powered solutions stay stable and react less with delicate ingredients. Every batch I’ve watched with this compound showed more predictability and less waste. Manufacturing plants know getting consistent performance pays off in reliability and compliance.
Potential Challenges and Smarter Use
No chemical comes without questions. Researchers ask tough questions about the long-term stability and toxicity of any new additive, this one included. While studies so far give this salt a good safety score compared to older choices, it pays to stay careful and double-check new data as bigger industries take it up. Academic groups and government labs now chase more evidence, looking for ways to recycle or neutralize spills, so progress won’t bring a new waste headache.
Moving Forward With Smarter Solvents
As manufacturers build out electric vehicles, grid batteries, and new industrial processes, attention will stay fixed on materials with promise. From my hands-on experience and the reading I trust, Tetrapropylammonium Bis((Trifluoromethyl)Sulfonyl)Amide isn’t just more safe—it’s helping solve bottlenecks in energy, cleaning, and precision manufacturing. With clear benefits in durability and safety, chemists now use it to bridge the gap between greener production and competitive results.
What Really Happens On the Shelf: Protecting the Chemical’s Integrity
I’ve handled enough specialty chemicals to know the challenges that arise from a busy lab bench and less-than-ideal storage. Tetrapropylammonium Bis((Trifluoromethyl)Sulfonyl)Amide is no exception. Tossing a bottle in a sample closet usually ends in trouble. This compound draws moisture from the air, a trait known as hygroscopicity. I’ve left similar salts open just a little too long, only to find a sticky mess the next week. If moisture gets in, clumps form and properties shift. It takes more time and money to salvage contaminated material — or you toss it outright. The main lesson? Keep it dry, keep it sealed.
Room Temperature Is Not Always Enough
Many researchers grab their chemicals and hope the cap holds. A glance at recent safety data sheets highlights the real risk. Tetrapropylammonium Bis((Trifluoromethyl)Sulfonyl)Amide prefers storage at room temperature or below. But lab “room temperature” can drift past 30 °C in summer. Heat speeds up decomposition for sensitive materials, ruining those crucial anion properties. I’ve seen the results firsthand: strange odors, color changes, disappointing yields. Small changes in environment eat away at experimental confidence. My basic rule keeps the bottle in a low-humidity, cool cabinet, far from sun-soaked windows or radiators. Simple tools, like silica gel packs and desiccators, work well. They go a long way in avoiding loss of investment — financial or scientific.
Keep Incompatibles Far Apart
Far too many accidents come from placing the wrong bottles side by side. For Tetrapropylammonium Bis((Trifluoromethyl)Sulfonyl)Amide, common sense prevails: keep acids, oxidizers, and open water away. A single spill or unexpected reaction triggers headaches and calls to hazardous waste cleanup crews. Chemistry isn’t just about what happens inside the flask; safe storage protects researchers, staff, and the surrounding environment. Colleagues sometimes underestimate the importance of segregation until a minor incident disrupts the whole day. Avoiding that stress just means reading the labels, checking the safety data, and keeping incompatible items apart.
Labeling and Documentation Matter More Than You Think
Good stewardship ties directly to proper labeling. A missing or faded label becomes a nightmare after a few months. I’ve spent hours sorting through mystery bottles without clear information. Accurate dating and logging — including where the sample sits and what conditions it’s seen — protects the next user and stops costly mistakes. Documentation supports traceability and aligns with best practices from organizations like OSHA and the ACS. Researchers can trace problems back to the source. This accountability carries more weight than many realize, especially under strict regulatory audits.
Real Benefits Beyond The Lab
Careful storage helps more than just the researcher working with the chemical. Reduced degradation means safer handling, cleaner disposal, and better outcomes for everything downstream. In my own experience, vigilance with storage protocols saved time, budgets, and — most importantly — health. Practicing good habits sends the right message to new lab workers: attention to detail pays off, both in data quality and safety. None of this takes fancy equipment, just discipline and respect for the substance on the shelf.
Understanding the Risks Behind the Chemical Name
Scan a lab supply list and find names that twist the tongue, none more complicated than Tetrapropylammonium Bis((Trifluoromethyl)Sulfonyl)Amide. Chemists know it as a component in ionic liquids—a class used in batteries, organic synthesis, and specialty solvents. The question lingers: just how risky or toxic is this stuff?
Digging Into What’s Inside
No one denies ionic liquids sound friendly—they don’t explode like gasoline, and they barely evaporate. Yet experience in chemical research makes one cautious. The trifluoromethyl sulfonyl group adds real bite. Trifluoromethyl compounds tend to resist breaking down in the environment. Once spilled, they stick around, seeping into water or soil, and proving tough to clean up.
The ammonium part drags concerns from my early lab days. Simple trialkylammonium salts often blur the line between “harmless” and “headache.” They can irritate skin, trigger allergic reactions, or worse, sneak through gloves and get under your skin after repeated use. The larger “tetrapropyl” tail slows absorption, yet no one claims complete safety.
Poking at Toxicity Data
Search hard and the safety data sheets show up less detailed than most would like. No large body of animal testing covers this compound. Hand-to-mouth exposure, splashing to the eyes, or inhalation shouldn’t be shrugged off. Several similar ionic liquids showed strong potential to mess with the liver or kidneys, backed up by rodent trials and cell studies. These “quaternary ammonium” compounds give labs reason to open the windows and pull on respirators.
There’s another angle. The fluorinated tail increases environmental persistence—think forever chemicals. Perfluorinated chemicals link to endocrine issues, immune problems, and sometimes cancer, which raises a red flag for anyone thinking about careless disposal or long-term buildup. It took years for regulators to catch up with PFAS, and the backlog built up in landfills, lakes, and bloodstreams worldwide.
Real-World Handling Speaks Volumes
In work environments, most labs treat Tetrapropylammonium Bis((Trifluoromethyl)Sulfonyl)Amide with care. Nitrile gloves, splash goggles, and chemical fume hoods become part of the daily routine. No one wants to find out about its risks the hard way. Colleagues warn about even tiny spills eating through bench coatings or leaving a lingering “chemical” smell long after a project finished.
Waste disposal rules normally demand more than dumping down the drain. Hazardous waste bins fill quickly—labelled for fluorinated organics and handled by professionals instead of grad students or new hires. Any shortcut taken often leads to fines, health complaints, or at worst, emergency calls to poison centers.
Fixing Gaps and Staying Safe
Regulatory bodies, including the EPA and ECHA, spend years catching up with new chemicals. For now, this one flies under the radar, largely due to its use outside consumer products. Pushing for more research helps fill knowledge gaps. Industry has the option to develop safer replacements or design solvents that break down after use. As more chemists share their experience openly, it gets easier to spot patterns, avoid risks, and demand better labeling.
Relying on good ventilation, personal protective equipment, and strict waste handling isn’t just best practice—it’s survival in the world of weird chemicals. From what I’ve seen, cutting corners just isn’t worth it when health and the planet are on the line.
Understanding the Formula
Tetrapropylammonium Bis((Trifluoromethyl)Sulfonyl)Amide, to keep things straightforward, comes with a mouthful of a name but a crisp chemical formula: (C3H7)4NNTf2. Breaking it down means looking at two main pieces. The tetrapropylammonium part—four propyl chains all hooked to a single nitrogen atom, creating a big, friendly cation: (C3H7)4N+. The anion, often recognized among chemists by its abbreviation "NTf2," consists of two (trifluoromethyl)sulfonyl groups bonded to a central nitrogen. Structurally, it’s written as [(CF3SO2)2N]-. The full structure ends up looking like a chunky puzzle with a positive side and a negative side, all tied up to give special qualities that labs and industry folks chase after.
Why Structure Matters
Chemistry classes hammer into students the idea that shape shapes function. Here, that big tetrapropylammonium ion makes the compound highly soluble in organic solvents, not something you'd easily see with small, simple salts like table salt. The NTf2- part gains attention because its electron-withdrawing groups (trifluoromethyl and sulfonyl) spread out negative charge, giving it remarkable stability. In plain talk, it's tough, it sticks around, and it doesn’t mess with the compounds it meets. This behavior opens doors for creative synthesis in organic labs and makes scaling up possible in the battery world or even pharmaceuticals.
The Role in Modern Research and Industry
Researchers turn to Tetrapropylammonium Bis((Trifluoromethyl)Sulfonyl)Amide for its unique set of tricks. For example, ionic liquids based on this compound stay liquid at room temperature, which makes them green alternatives for toxic or volatile solvents. Instead of spilling harmful vapors into the air, these liquids just sit there—stable, non-volatile, reusable. This matters with big conversations swirling around sustainable chemistry and waste reduction. Electrochemists love this ionic liquid pairing; it brings low viscosity and excellent ionic conductivity, two prerequisites that drive better fuel cells and safer batteries.
I’ve seen researchers hit all sorts of walls with degradation and impurity buildup using more reactive salts. In contrast, using NTf2--based salts, cleanup turns easier, and endpoints show clearer data. Teams in environmental labs point out fewer emissions in their air monitoring when swapping to these ionic liquids. This shift feels like a small step, but in a field where waste and safety drive regulations, any way to get chemicals with fewer side effects packs a punch.
Challenges and Looking Forward
No chemical wins every category. Tetrapropylammonium Bis((Trifluoromethyl)Sulfonyl)Amide comes with its own set of headaches. Making NTf2- anions uses fluorine-heavy reagents, which don’t do the planet any favors. Environmentalists have pushed for scrutiny over fluorinated compounds, especially as persistent organic pollutants show up far downstream. Scientists started tinkering with milder synthesis steps or chasing entirely new anions that deliver similar perks with fewer environmental strings attached.
Labs can also look for recycling schemes. Recovering and reusing these special salts cuts down on waste and lowers the chemical footprint. Open sharing of synthesis routes, purity tests, and real-world safety data might sound tedious, but it has sharpened the focus and knowledge of everyone in the room. My work always circles back to community trust—sharing pitfalls and publishing unexpected results gives industry and academia real tools to solve tomorrow’s problems.
Tetrapropylammonium Bis((Trifluoromethyl)Sulfonyl)Amide may not become a household name, yet its mark stretches across clean technology and green chemistry circles. For practitioners and policymakers alike, attention to detail in structure and use marks the difference between legacy pollution and future solutions.
What Makes This Salt Stand Out
Tetrapropylammonium bis((trifluoromethyl)sulfonyl)amide—let's just call it TPATFSI for short—fills a pretty specific role in the world of ionic liquids and specialty solvents. The unique cocktail of the tetrapropylammonium cation and the bis(trifluoromethyl)sulfonyl)amide anion gives TPATFSI some unusual, even handy, solubility properties. After seeing this stuff used in both academic labs and industry, I can say you get an appreciation for just how different it acts compared to your typical organic salts.
Water Isn’t Always the Answer
TPATFSI barely bothers with dissolving in water. Those fluorinated sulfonyl groups push away water molecules, leaving this salt almost intact in an aqueous solution. You can try swirling it around or heating your flask, but most of the salt stays clumped at the bottom. This goes against what a lot of folks expect, especially if they’re used to more common ionic compounds, where water usually wins the battle.
In practice, this low water solubility makes TPATFSI pretty useful in non-aqueous electrochemistry. You won’t have to worry about water interfering with your measurements or messing up your sensitive reactions. Researchers working on lithium-ion batteries or supercapacitors often reach for salts like this. They want something that keeps the electrolyte dry, stable, and wide-open to ions that matter, like lithium or sodium.
Solvents Where TPATFSI Comes Alive
If you toss TPATFSI in something with a bit of backbone, like acetonitrile, dimethyl sulfoxide (DMSO), or even propylene carbonate, it acts much differently. Things dissolve fast and thoroughly. Organic solvents with high polarity or with an affinity for big, chunky ions really make a difference. In plain language, in organic or aprotic media, you get all the benefits of TFSI's massive anion without the headaches of separation or precipitation.
A lot of advanced battery development relies on testing these kinds of salts in ever-stranger solvents. I’ve watched colleagues try every blend they could think of in pursuit of just a few extra cycle lives out of their cells. The way TPATFSI slips into these exotic environments shows how far electrochemical science has moved from the days of water and sodium chloride.
Environmental Considerations
Fluorinated compounds like TFSI anions don’t vanish in nature. They can linger, just like PFAS chemicals that get plenty of headlines these days. Some labs collect every scrap of waste TPATFSI for careful disposal, keeping spills to a minimum and documentation tight. Environmental health researchers have started watching these salts more closely, especially since very little is known about their long-term fate. This creates some tension between the excitement of new materials and the real-life need to avoid lasting pollution.
Room for Safer Alternatives?
A lot of solid research focuses on finding salts with a similar mix of stability and solubility, without the permanent environmental footprint. Approaches include swapping out fluorinated anions for more biodegradable options or developing recycling programs focused on closed-loop lab systems. The chemistry community has seen promising starts, but good replacements need to match the powerhouse performance of TFSI salts. Full adoption will take time, trial, error, and persistence.