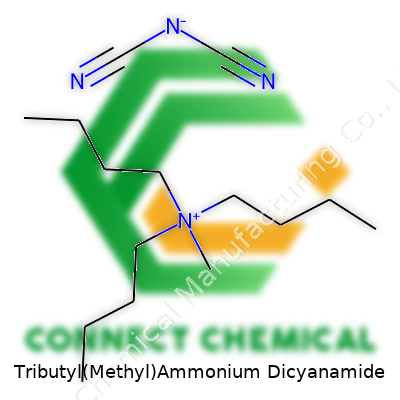
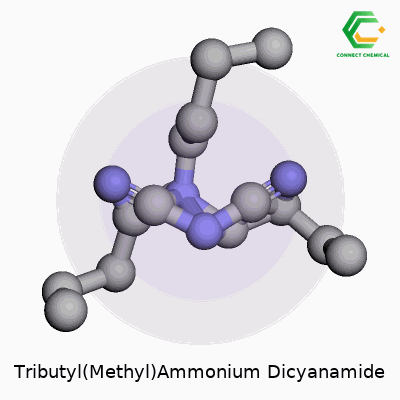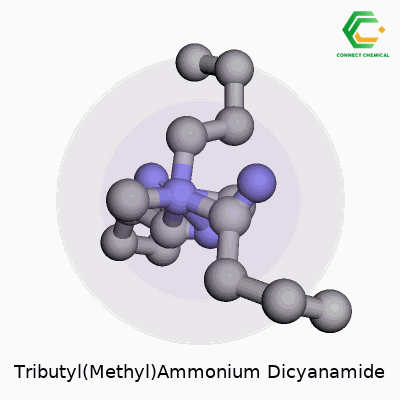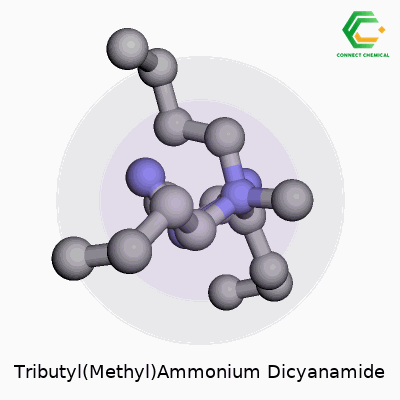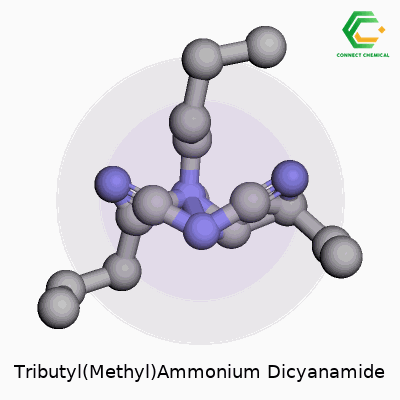
Tributyl(Methyl)Ammonium Dicyanamide: A Commentary
Historical Development
Chemistry often moves forward through collaboration and curiosity, and the story of Tributyl(Methyl)Ammonium Dicyanamide reflects both. Early on, researchers recognized the utility of quaternary ammonium compounds, and as industries demanded greener, more tunable ionic liquids, research teams began tweaking established frameworks. The journey toward dicyanamide-based salts emerged from experimentation with anions that could offer better thermal stability and less volatility. I remember poring over journals where the shift toward room-temperature ionic liquids pushed chemists to combine new counter-ions with established quaternary ammonium cations. The mid-to-late 20th century marked a wave of interest in such molecules, driven by needs in electrochemistry and catalysis. By the time the first commercial syntheses made prices more accessible in the early 2000s, academic groups were already benchmarking new salts like Tributyl(Methyl)Ammonium Dicyanamide for conductivity, low-flammability, and compatibility with sensitive systems.
Product Overview
Tributyl(Methyl)Ammonium Dicyanamide falls in the family of ionic liquids, a class valued for their low volatility and ability to dissolve an impressive range of organic and inorganic substances. In my experience with laboratory synthesis, this compound’s ease of handling and broad compatibility simplifies complicated processes that used to require several different solvents and buffers. The dicyanamide anion offers more than just a counter-ion; it brings unique coordination behavior and can influence thermal and chemical stabilities. The product often appears as a clear to slightly yellowish liquid at room temperature, with a faintly amine-like smell. Product catalogs from major suppliers emphasize features like high thermal stability, wide liquid range, and electrical conductivity that meet the requirements of most research and industrial projects. Commercial grades offer varying purities and pack sizes, letting labs scale from academic research to pilot-plant batches.
Physical & Chemical Properties
Physical and chemical characteristics shape how a chemical influences both the lab bench and the factory floor. Tributyl(Methyl)Ammonium Dicyanamide retains a low melting point, generally keeping its form as a viscous liquid under standard conditions, which helps for vacuum transfer and pipetting without special containment. Its density hovers around 0.95 g/cm3, about the same as water, but it feels slicker and leaves a residue if spilled—nothing dangerous, but definitely sticky. The ionic nature grants the liquid electrical conductivity in the range useful for some battery applications. In the lab, I have seen robust stability across a wide pH range, although strong oxidizers or acids can fragment the molecule. The cyano groups in the dicyanamide anion tolerate high heat, so decomposition temperatures above 200°C make process engineers more comfortable when scaling up. Water miscibility depends on specific formulation—water can solvate both the cation and anion, but too much can hydrolyze or shift equilibria. Chemical suppliers often report detailed spectra and chromatograms to assure researchers the absence of impurities that might poison catalysts or interfere with analyses.
Technical Specifications & Labeling
On the packaging side, clear, durable labels typically list the IUPAC name, CAS number, batch ID, and storage guidance. Labels need to highlight water sensitivity, any incompatibilities, and recommend temperatures for storage—usually between 15 and 30°C. Lab users have to check for regulatory flags—a dicyanamide anion can sometimes fall into restricted lists in regions concerned about cyano compounds, so legal teams double-check shipping paperwork. Certification sheets must show compliance with REACH, TCSA, or regional chemical inventories, so researchers don't get tripped up by customs. Product data sheets provide spectral data (NMR, IR, MS) to confirm purity, and QA/QC teams track lot-to-lot consistencies. Technical specifications outline minimum purity thresholds—often above 97% for research grade, along with color, water content, and Halide tests to rule out side products from quaternization reactions. Handling and disposal instructions, as well as emergency first aid information, appear on both the data sheets and primary containers.
Preparation Method
Manufacturing Tributyl(Methyl)Ammonium Dicyanamide starts with quaternization of tributylamine using methylating agents, usually methyl chloride, under strictly controlled conditions to create the quaternary ammonium cation. The reaction needs careful temperature management and inert atmosphere to prevent side reactions. Once the cation is produced as a halide salt, which can be isolated as a solid or viscous liquid, anion exchange takes center stage. Dicyanamide salts, typically sodium or potassium dicyanamide, react with the ammonium halide in an aqueous or biphasic system. Filtration and washing steps remove inorganic byproducts. The resulting ionic liquid separates and is dried using vacuum evaporation or molecular sieves. In practice, drying ensures the compound’s stability and performance in downstream uses—water and halide residues would otherwise compromise electrochemical or catalytic activity. Many commercial processes have automated these steps for larger scale, safely managing toxic reactants and ensuring batch reproducibility.
Chemical Reactions & Modifications
Tributyl(Methyl)Ammonium Dicyanamide stands up well to many chemical environments but also participates in interesting transformations. The dicyanamide anion can serve as a ligand, coordinating to metal centers and forming stable complexes, which opens doors in homogeneous catalysis. Under strong acid, hydrolysis can fragment the anion, producing ammonia and cyanide derivatives—hazards that call for ventilation and containment. From personal experience, its cation resists alkyl transfer, leaving most of the chemistry focused on the anion’s behavior. In organic synthesis, the ionic liquid can enhance reaction rates by stabilizing transition states or solvating ionic intermediates. Modifying the cation by swapping one alkyl chain for a longer or branched group adjusts viscosity and hydrophobicity—chemical companies often customize the cation for clients needing specialty properties. Researchers have tested dicyanamide-based ionic liquids with embedded task-specific groups, tailoring reactivity for CO2 capture or metal extraction applications.
Synonyms & Product Names
This compound answers to several names, with suppliers and researchers adopting different conventions. Commonly encountered synonyms include N,N,N-tributyl-N-methylammonium dicyanamide, tributylmethylammonium dicyanamide, and several shorthand letter-number codes in supplier catalogs. Standard referencing sticks with both the quaternary ammonium structure and the dicyanamide anion, so anyone involved in procurement or research must cross-check the structural formula and CAS registry number to avoid mixups with similar ammonium salts or professional-grade ionic liquids. Some producers market it under proprietary blends, either pure or with specified water content, which highlights the need for clear ordering and communication when purchasing for regulated applications.
Safety & Operational Standards
Safe handling deserves constant attention, as both the cation and anion can present health or environmental hazards. Contact with skin or eyes brings irritation, so even though I’ve seen minor exposures in the lab lead only to redness or dry skin, I never work without proper nitrile gloves and splash goggles. Inhalation risks arise when the compound mists or vaporizes, which underscores the value of chemical hoods and local exhaust ventilation. Storage in tightly sealed containers safeguards against moisture and keeps the liquid clear and free from hydrolytic breakdown. Labs should post warning signs and keep spill kits close—cleanup of dicyanamide compounds benefits from dry absorbents and immediate collection to prevent environmental release. Safety data sheets recommend monitoring indoor air quality for any released cyano fragments or vapors. Emergency protocols must stay up-to-date, with first aid guidance on treating skin or eye contact using plenty of water and seeking medical attention for inhalation symptoms. Waste collection follows local hazardous chemical regulations—residues or wash solutions go into compatible containers for controlled incineration or chemical neutralization, not down the drain.
Application Area
Tributyl(Methyl)Ammonium Dicyanamide draws attention from a wide array of fields. Electrochemists pursue it as a solvent or supporting electrolyte for batteries and capacitors because of its wide electrochemical window and non-flammable nature—qualities not found in more volatile organic solvents. In extraction chemistry, the ionic liquid supports metal ion separation and recovery, often outperforming older, less selective extractants. Organic synthesis labs value the compound’s ability to boost nucleophilic substitution rates and solubilize a broad range of reactants. Within catalysis research, its polar but non-aqueous environment supports new reaction pathways, helping create specialty chemicals or pharmaceuticals cleanly and efficiently. From firsthand work in pilot-scale operations, I have seen engineers trial the compound as a dispersant or anti-static agent in formulations that would gum up or short out with other materials. Researchers push its boundaries, examining how dicyanamide salts stabilize biomolecules or enable green chemistry transformations.
Research & Development
Innovation in the field of ionic liquids marches forward relentlessly, with Tributyl(Methyl)Ammonium Dicyanamide often at its forefront. Academic labs publish new protocols that unlock faster syntheses or cleaner reaction conditions using the compound. Companies test it in separations of rare earth elements—a critical step for sustainable technology supply chains—replacing more harmful solvents. Multidisciplinary teams join chemists and engineers to expand the list of viable reactions, pushing ionic liquids into pharmaceutical synthesis, CO2 capture, and recycling processes. Dozens of patents have issued covering tweaks to either the cation or anion structure, showing just how customizable the field has become. Conferences highlight ways the compound helps solve technical bottlenecks in emerging technologies like battery electrodes or sensors. Still, processing costs and sourcing sustainable feedstocks remain hurdles before full industrial adoption—R&D projects tackle these with new catalysis designs and process intensification efforts.
Toxicity Research
Understanding and mitigating toxicity stands at the core of moving new chemicals toward practical, widespread use. Toxicology screenings reveal that Tributyl(Methyl)Ammonium Dicyanamide, like many quaternary ammonium salts, irritates mucous membranes and can impact aquatic life with improper disposal. Animal studies show low acute toxicity by ingestion, but longer-term exposure can present risks, particularly to the liver and kidneys, calling for strong stewardship during scale-up and disposal. In my review of recent regulatory filings, many government agencies now flag dicyanamide-based products for strict environmental controls, especially due to the potential for cyano group breakdown products to bioaccumulate or cycle through water systems. Researchers explore degradability and run bioassays on soil and water samples to map any lingering effects. These findings press companies to refine containment and recycling protocols, in order to satisfy both environmental watchdogs and community health standards.
Future Prospects
The outlook for Tributyl(Methyl)Ammonium Dicyanamide holds promise tied to both technological trends and regulatory realities. As industries chase safer, lower-carbon alternatives for electronics, energy storage, and high-value manufacturing, dicyanamide-based ionic liquids could fill critical gaps left by traditional solvents. Next-generation batteries, fuel cells, and solar panels all stand to benefit from safer electrolytes, and manufacturers draw up pilot projects to integrate these ionic liquids into working prototypes. Universities run long-term life-cycle studies, in which durability and recyclability shape the chemical’s odds of mainstream adoption. Regulatory frameworks evolve alongside technical progress—production processes designed for lower waste and closed-loop recycling satisfy both economic and environmental pressures. I see future breakthroughs coming not only from incremental chemistry but from supply chain integration, collaborative safety research, and smart regulation that encourages cleaner, smarter products. As researchers untangle remaining questions on toxicity and scalability, the path toward expanded use keeps opening up for this class of compounds, setting the stage for greener and more innovative chemistry worldwide.
Unique Properties Drive Real-World Uses
Tributyl(methyl)ammonium dicyanamide isn’t the sort of chemical you hear about at dinner parties, but its role in modern industry runs deep. So what makes it stand out? The ionic structure of this compound brings a mix of good solubility, low volatility, and strong thermal stability. These features open up some very practical uses, especially for folks in advanced materials and chemistry circles.
Solvent and Electrolyte in Batteries
Battery development keeps moving at a rapid pace, and this compound steps into some of the latest research. Lithium-ion and sodium-ion batteries both benefit from ionic liquids that don’t catch fire and can boost performance. Tributyl(methyl)ammonium dicyanamide has shown promise as an electrolyte or electrolyte additive because it remains stable at high temperatures and lowers the risk of hazardous reactions. By extending battery life and improving safety, it answers some long-standing challenges in energy storage.
Polymer and Material Science
Making high-performance plastics and composite materials often calls for a chemical nudge in the right place. This dicyanamide-based compound acts as a catalyst or plasticizer, depending on the job. It helps control the curing process of epoxy resins, which are used in everything from airplane parts to circuit boards. In some cases, it brings more flexibility to finished materials, which can save manufacturers money and trim waste during production. High-quality polymers go into smartphones, car interiors, and sports gear, making the compound’s benefits land where folks might not expect.
Green Chemistry and Catalysis
Chemical processes run better with the right catalyst, and many researchers have turned to ionic liquids as a cleaner choice instead of old-school organic solvents. Tributyl(methyl)ammonium dicyanamide stands out because it works under milder conditions and cuts environmental impact. By trimming volatile organic compound emissions, it matches new regulations in Europe and North America. Experts working on pharmaceuticals and specialty chemicals can use this compound to bump up yields without leaving a heavy pollution footprint.
Pharmaceutical Synthesis
Drug-making often follows tightly controlled steps, with small mistakes costing a fortune. Scientists value dicyanamide-based ionic liquids for helping tailor certain synthesis reactions. The compound’s unique chemical background offers more flexibility in choosing reaction partners and conditions. This can speed up development and even open doors to new kinds of medicines that might not work using older techniques. In this way, Tributyl(methyl)ammonium dicyanamide plays an indirect role in modern healthcare progress.
Next Steps: Tackling Challenges
While the benefits stand tall, it doesn’t solve every problem in chemistry. Handling and disposal always matter—this compound needs proper care to stay safe and green. Waste treatment and recycling protocols are under review to help reduce the impact once it leaves the lab. The push for safer, more sustainable chemicals fuels further study. Researchers want to understand its long-term health effects, improve reusability, and keep costs reasonable. Conscientious choices and open communication help integrate these chemicals responsibly, so benefits reach the marketplace without overlooking safety.
Looking at the Name: Tributyl(Methyl)Ammonium Dicyanamide
Many of us who spent time in a chemistry lab have come across mouthfuls like “Tributyl(methyl)ammonium dicyanamide.” The name sets off a chain of images—big hydrocarbon arms, nitrogen-heavy partners, and a story of ions coming together. The compound brings together an organic ammonium cation and an anionic part known for its two cyano groups. Chemists often land on this salt because it blends hydrophobic alkyl chains and an electronegative anion. So, what’s in this big-sounding phrase?
Breakdown of Structure and Formula
The cation starts with the ammonium center. Here, “tributyl” tells us there are three butyl groups—strings of four carbon atoms—attached to the nitrogen. Add a “methyl” and you’ve got the fourth substituent: a simple one-carbon add-on locked onto that same nitrogen. The nitrogen core holds a positive charge because each of these side chains replaces what would be hydrogen in a simple ammonium ion.
Chemists write the formula for this cation as N(C4H9)3(CH3)+. If you’ve worked with these before, you’ll know butyl groups bring bulk, making the molecule greasy compared to simpler salts. The cation balances a dicyanamide anion, which formula-wise is N(CN)2–. Here, the “dicyanamide” signals two CN groups flanking a nitrogen. Lay them out, and you get a resonance-stabilized anion promising chemical stability and some interesting reactivity with metals.
Pair the two and the compound’s formula becomes N(C4H9)3(CH3)N(CN)2. Each segment contributes something—alkyl chains steer solubility and thermal properties, while the anion gives the molecule a strong electronic punch.
Structure in Living Color
Put the pieces together, and you’ll see this: the nitrogen at the core of the cation forms four single bonds out to three butyl groups and a methyl group. It looks pretty crowded, and in three dimensions the butyl arms splay outward as far as possible. The dicyanamide, with its linear arrangement of C and N atoms, pushes up against the big cation. In reality, the full structure would need a diagram, but chemists picture a “big-ball, little-ball” pairing. The bulkier the cation, the more it can change how the salt behaves compared to a smaller ion like ammonium.
Organic chemists value salts like this for their role in “ionic liquids”—substances that stay liquid at lower temperatures and can dissolve a whole spread of compounds. The dicyanamide anion doesn’t bind metals too tightly, which gives room for catalytic or separation work, a need that pops up in fields like electrochemistry or green extraction methods.
Why Chemical Structure Matters
I’ve handled these sorts of compounds during graduate research. Handling organic ammonium salts reminds you just how much physical structure affects the experience. Bulky alkyl groups reduce melting points, making some of these salts semiliquid at room temperature. The large, soft cation resists crystallizing into a simple lattice—an advantage for anyone looking to avoid solids plugging up a process line.
It’s also worth noting that not every organic salt gets treated equally in safety data. The butyl groups raise questions about bioaccumulation, while the dicyanamide motif prompts investigation into breakdown products under heat or acid. Some labs in Germany published breakdown studies showing dicyanamide decomposing to cyanamide and eventually to ammonia and cyanide under certain conditions, a stark reminder of the need for proper handling.
Practical Use and Responsible Chemistry
Tributyl(methyl)ammonium dicyanamide stands out in research for its tunability: swap out the alkyl groups or shift the anion and a new set of properties appear. Industries have found use in greener solvents, heat transfer agents, and occasionally as phase-transfer catalysts. Responsible use requires clear labeling, correct PPE, and a good eye on waste stream management—small differences in chemical structure shift the safety landscape.
Every new salt gives a toolkit for research and production. Understanding the chemical structure of compounds like this makes sure we balance innovation and safety in labs and industry alike.
Understanding What We're Dealing With
Some chemicals have names that fill a mouth and rattle your nerves all at once—tributyl(methyl)ammonium dicyanamide is one of those. Anyone who's ever worked in research or helped manage manufacturing knows these aren't burger toppings. This chemical steps onto the scene as part of ionic liquids, that trendy family of salts turning heads in labs for their use in battery development, catalysis, and industrial processes. Cool-sounding, but is it safe to breathe around? Or touch with bare hands? These are not silly questions; they come up any time folks run into something from a supply drum marked with more syllables than instructions.
What The Data Says: Risk and Reality
Whenever I dig through safety data sheets, the real test is: does this stuff cause burns, lung issues, or toxic shocks? A few facts stand out. Tributyl(methyl)ammonium dicyanamide doesn't belong to the heavyweights like mercury salts or cyanides, but it brings its own quirks. Acute toxicity ratings don’t put it in the same league as bleach or pesticides, yet lab tests hint at skin and eye irritation. No one wants to find out firsthand. On contact, it can cause redness or rashes. People with sensitive skin don’t fare well. Most data points toward low vapor pressure, suggesting inhalation hazards aren’t as pressing, unless you heat it or handle big quantities in a small, stuffy room.
What’s less obvious: breakdown products. The dicyanamide part includes hidden risks, especially under bad storage or disposal. Heating or incineration can produce hydrogen cyanide—a notorious poison. Inexperienced users sometimes shrug, but anyone who's worked with chemicals like this stays alert. If the fire alarm blares in a warehouse storing dicyanamide compounds, nobody wants to stick around for the smoke.
Safety At Work and Home
Real world use of this chemical happens in places with rules and procedures. Lab coats, gloves, goggles—all the everyday shields for handling. Fume hoods are routine in proper research setups. Once you step outside these boundaries, things can get dicey. I’ve watched people skip gloves for “just a minute” and regret that decision the rest of the week.
Large spills in the workplace deserve respect, not bravado. Dry chemicals like this can dust up and settle in corners, waiting for someone to accidentally drag it onto their phone or face. Cleanup isn’t simply about a mop and bucket. Disposal works best with experienced staff who know not to flush it or toss it in regular trash. Following the global harmonized system (GHS) for chemicals leads to fewer accidents.
Solutions That Make Sense
Nobody thrives on fear, but ignorance weighs heavier. Simple steps matter most. Train everybody who touches chemicals like this; don’t stand for mystery labeling or absent safety sheets. Facilities should invest in proper ventilation and keep spill kits stocked. Regulations require more than paperwork—they’re lessons built from hard-lived mistakes. Current practices favor substitution with safer alternatives where possible, especially in education or pilot studies.
It’s on everyone—the company, researchers, managers—to respect what’s in the drum, not guess at its mood. Mistakes come not just from disaster, but from boredom and rushing. The more everyone pays attention, the less likely a scary moment will sneak up on the next shift. Making chemical safety part of routine, not a once-a-year chore, keeps both factories and homes out of the headlines.
On the Ground: Recognizing the Substance
Tributyl(Methyl)Ammonium Dicyanamide sits on the shelf in some labs as a specialty chemical, often stepping in for ionic liquids or as a catalyst in organic synthesis. It’s not something you spot every day, but if you do work with it, you have to know what you’re dealing with—this material offers significant convenience, yet it does introduce risks if not managed with respect.
Keeping Storage Simple and Safe
Anyone who’s spent time in a decent lab can spot trouble with bottles left open or chemicals stacked without care. This compound definitely shouldn’t wind up among neglected bottles. It absorbs moisture from the air, and that can change how it behaves. For long-term safety, a cool, dry spot with decent ventilation makes sense. I’ve worked in spaces that relied on old metal cabinets away from direct sunlight and heat—the basics don’t go out of style.
Tightly sealing containers is no small matter. I once watched a half-open container pick up moisture and clump together, which caused dosing headaches and clean-up nightmares. If glass works in your lab, keep it there, though quality plastic with chemical resistance does a good job too. Chemical storage should never brush up against food, drink, or any shared personal spaces. In my experience, label everything by name, date, and hazard.
Hands-On Handling: Where Small Steps Prevent Big Trouble
I’ve watched rookie mistakes add up quickly with substances like this—no gloves, a splash, and then a scramble for the sink. Good gloves (usually nitrile), protective eyewear, and a sturdy lab coat make a difference. Lab workers need to watch themselves and each other. Getting lazy with personal protection doesn’t end well.
Transfer work in a fume hood or under exhaust fans, especially if you get dust, vapor, or splashes. The odor may not give much warning, but the risks are real. Never pipette by mouth or rush an experiment just to save time. For spills, a step-by-step approach works best: contain, clean, and dispose of the waste according to local rules. Even a few grams on a bench can mess up the rest of the day.
Disposal Shouldn’t Be an Afterthought
Hazardous waste bins exist for a reason. Dumping this chemical into drains or regular trash brings both legal and health consequences. In one case, a local regulator traced back environmental contamination to mishandled cleanups—it only took a few slips for fines and audits to arrive. Nobody wants that.
Bag contaminated wipes, gloves, or anything else exposed to the compound and ship these out with chemical waste according to the site’s protocol. Take pride in doing it from start to finish; experience says it adds up to cleaner, safer work for everyone.
Building Good Habits
Consistent training gives peace of mind. Read the Safety Data Sheet and keep it within reach; regulations shift, so update as new data arrives. Run periodic safety reminders, and make sure the new folks watch and learn. I’ve seen plenty of incidents avoided just by reviewing a checklist or walking through a dry run before actual handling.
Storage and handling sound like routine chores. They’re actually where professionalism shows. Paying attention to details today prevents disaster, downtime, and regret tomorrow. Handle with care—you and your team will feel the difference.
Understanding the Real-world Benchmark for Purity
Tributyl(methyl)ammonium dicyanamide does not pop up in daily conversation outside the lab, but for researchers and chemists, understanding its purity matters more than most realize. Labs depend on the certainty that each bottle has been tested to a high standard—usually 97% to 99% purity. The difference between hitting 97% and pushing closer to 99% comes down to what the end-user values: cutting costs or guaranteeing results.
On paper, manufacturers pitch this compound with purity specs hitting at least 98%. The Certificate of Analysis gives an actual value, sometimes even nudging toward 99% if the process and starting materials are handled right. What stands out is that manufacturers often include more than just the number—water content, color, specific impurities, and residual solvents come along for the ride. That certificate isn’t just a boring formality; it’s the one thing labs use to avoid sabotaging a sensitive reaction with mystery contaminants.
Personal Experience and Why Purity Matters
I’ve seen more than one ambitious project stall because someone grabbed a bottle without checking the data sheet. The trouble comes from compounds like tributyl(methyl)ammonium dicyanamide being sensitive to traces of water or leftover reactants—they creep in during synthesis and sneak into the reaction, dragging yields down or throwing results into chaos. Skipping a careful check on those Certificate of Analysis details means betting on luck instead of science.
Most suppliers like Sigma-Aldrich, Alfa Aesar, or TCI America offer their best grade with at least 97% purity stated upfront. HPLC or NMR verification backs up this claim, cutting down the guesswork. Reputable suppliers will lay out impurity profiles, sometimes showing less than 1% water or related ions, because even tiny levels easily spoil reactions with strict stoichiometry.
Verification, Transparency, and Trust
Trust in chemical supply chains hasn’t come from big branding. Labs build trust by double-checking each batch, running their own NMR, or IR if the stakes are high. Some research groups I’ve worked with keep backup batches from separate suppliers, just in case those subtle trace impurities pop up during quality control. It’s not about paranoia—no one wants weeks of work torpedoed by a hidden contaminant.
Transparency matters, and the best suppliers make it easy to get batch-level documentation. Every time I order, I want a data trail: not just the purity number, but the test method, batch code, expiration, and all known contaminants. Without that, purity numbers are just marketing.
Better Systems, Safer Research
Making the chemistry world safer and more predictable means suppliers committing to clearer documentation and buyers using those reports. Chemical companies could align testing standards internationally to cut confusion for teams working across borders, especially for substances like tributyl(methyl)ammonium dicyanamide where results can hinge on the tiniest impurity. My advice for anyone picking chemistry suppliers: don’t just ask for purity—demand the backstory to every number. Suppliers that respond willingly stand out, while silence usually signals something to hide.