Tributylhexylphosphonium Bis((Trifluoromethyl)Sulfonyl)Imide: A Closer Look
Historical Development
The road leading to the creation of ionic liquids like Tributylhexylphosphonium Bis((Trifluoromethyl)Sulfonyl)Imide has stretched over the better part of a century. In the 1970s and 80s, chemical researchers began to recognize that these salt compounds could exist as liquids at room temperature, setting them apart from traditional molten salts. By the turn of this century, attention shifted to phosphonium-based ionic liquids due to their enhanced stability compared to their ammonium and imidazolium relatives. This shift sparked a surge in synthesizing new variants like Tributylhexylphosphonium, which drew notice for its unique properties and promising role in green chemistry. Over the last two decades, industry and academic institutions collaborated to refine its production, which led to reliable protocols for scale-up and safer handling practices.
Product Overview
Tributylhexylphosphonium Bis((Trifluoromethyl)Sulfonyl)Imide, often known as [P4446][NTf2], landed on the scene as an answer to growing demands for safer, more robust ionic liquids. Unlike earlier types, it tolerates wider temperature swings and resists breakdown in the presence of water. It stands out with its low volatility and high chemical inertness, attributes that stem from the interplay between the bulky phosphonium ion and the delocalized charge of the NTf2 anion. As such, it became a go-to pick for applications ranging from electrochemistry to solvent extraction—places where its non-toxic and low-flammability nature offer clear rewards.
Physical and Chemical Properties
As a colorless to pale yellow liquid at room temperature, [P4446][NTf2] boasts a melting point usually below -10 ºC. Its density hovers around 1.15–1.20 g/cm³, higher than that of water yet within reach for standard organic solvents. Its viscosity, although higher than traditional organic liquids, grants useful control in process industries that favor slow diffusion and reduced volatility. Hydrophobic behavior sets it apart; it shuns mixing with water, which not only streamlines recovery but also limits leaching into the environment. Electrical conductivity remains modest here, making it suitable for use in systems designed for moderate ion transport. Thermal stability persists up to roughly 300 ºC, handsomely beating older ammonium analogs.
Technical Specifications and Labeling
Suppliers label [P4446][NTf2] with its CAS number, precise molecular formula (C22H43F6NO4P·S2), and batch-specific purity, which generally runs above 98%. Chemists count on supplier data sheets to outline water, halide, and acid content, each carefully controlled through purification methods such as column drying or azeotropic distillation. Most bottles arrive with hazard icons indicating its status as an eye and skin irritant, and shipping containers feature tamper-evident caps to uphold quality. Documentation provides spectral analysis (NMR, FTIR) and sometimes ionic conductivity tests, building trust for those working in regulated labs.
Preparation Method
Typical synthesis of Tributylhexylphosphonium Bis((Trifluoromethyl)Sulfonyl)Imide kicks off with a straightforward quaternization: Tributylphosphine reacts with 1-bromohexane, yielding the corresponding phosphonium bromide. The story continues with a metathesis step, swapping bromide for the NTf2 anion via reaction with lithium bis(trifluoromethanesulfonyl)imide in an appropriate organic solvent. After rigorous washing and drying, the product emerges as a low-viscosity liquid. Each step must fend off contamination—especially water or halide residues—since these can scuttle both stability and physicochemical properties. Careful solvent selection and glassware prep play a decisive role here, and scale-up labs run these sequences in dryboxes or under nitrogen blankets to shield from atmospheric moisture.
Chemical Reactions and Modifications
[P4446][NTf2] stands up to harsh environments, and classic ionic substitutions rarely succeed in prying away its stable NTf2 group. Yet, this compound welcomes functionalization at the phosphonium site, offering a platform for tuning the liquid’s solubility or hydrophobicity. Researchers have explored swapping out alkyl chains, lengthening or branching them to alter viscosity and melting points for specific industrial uses. These tweaks open the door to designer ionic liquids—chemically similar but precisely matched to each application. It resists unwanted side reactions, so long as care goes into shielding it from strong nucleophiles or acids that threaten the phosphonium core.
Synonyms and Product Names
Chemists refer to this compound in shorthand forms like [P4446][NTf2]. Elsewhere, it turns up as Tributyl(n-hexyl)phosphonium bis(trifluoromethylsulfonyl)imide or simply Tributylhexylphosphonium NTf2. Supply catalogs sometimes mark it by brand-specific codes or catalog numbers, yet the essential identity always pivots on the phosphonium and NTf2 coupling. Other labels, such as ionic liquid 220 or IL-PH-NTf2, may surface, but the reliable structure and properties keep it easily recognizable in technical circles.
Safety and Operational Standards
Safer than many organic solvents, [P4446][NTf2] still merits respect during handling. Lab practice revolves around gloves, goggles, and good ventilation, avoiding skin or eye contact that might cause irritation. Its low volatility reduces inhalation risks, yet accidental splashes call for fast cleanup—its stubborn staying power can cling to surfaces and resist simple water rinsing. Disposal takes its cue from environmental safety protocols, with used material captured for professional incineration or chemical breakdown. Industrial sites benefit from clearly labelled bottles, safety sheets readily on hand, and regular staff briefings to limit the odds of skin dermatitis or accidental oral exposure. Long shelf life and thermal robustness reduce fire risk, situating it ahead of many common solvents for routine use in lab and pilot plant settings.
Application Area
Wide-ranging applications mark the story of Tributylhexylphosphonium Bis((Trifluoromethyl)Sulfonyl)Imide. Electrochemical cells harness its broad electrochemical window to enable long-cycle batteries and capacitors. Its chemical inertness advances the separation of rare earth metals from aqueous streams, critical in recycling tech. Organic synthesis picks up on its non-coordinating character, deploying it to support catalytic processes without scavenging reactive intermediates. Because it barely evaporates, it cuts down on environmental loss in extractive metallurgy and solvent recycling. Researchers trial it in carbon capture, where its selectivity for CO₂ matches the push for more sustainable industrial practices. Companies with a green ethos tout its drop-in potential, using it to replace more hazardous VOCs without trading away efficacy or reliability.
Research and Development
Ongoing projects probe the edges of [P4446][NTf2]'s capabilities. Institutions compare its lifetime in demanding electrochemical applications to traditional acetonitrile and propylene carbonate mixtures, chasing higher energy density with longer shelf stability. Material scientists measure its influence on nanostructured catalysts, hunting for synergy between inert solvents and active metals. In the field of analytical chemistry, teams investigate novel solvent extraction protocols to minimize environmental release, using high-throughput screening to narrow down optimal conditions. Demand from green chemistry—especially for recyclable, low-toxicity solvents—keeps research well-funded. Meanwhile, advances in computational modeling search for ways to predict property changes from further tweaking of alkyl or anion substituents, potentially outpacing synthesis bottlenecks in the lab.
Toxicity Research
Safety by design only works if toxicity data stays transparent and robust. Studies place [P4446][NTf2] low on acute toxicity scales, with LD50 values substantially higher than for classical organic solvents. It largely resists bioaccumulation, though its breakdown products under strong oxidative conditions have raised some questions in aquatic toxicity models. Chronic exposure data remains limited, with research groups running tests on cell cultures and zebrafish models to clarify long-term impacts. Careful monitoring during pilot plant use reveals infrequent cases of dermatitis in sensitive personnel, but nothing compared to the hazards from legacy solvents like benzene or toluene. By keeping the conversation active about routes of exposure, washout procedures, and next-gen containment, industry teams uphold a consistent path toward safer adoption.
Future Prospects
The search for sustainable, high-performance materials drives ongoing interest in ionic liquids like Tributylhexylphosphonium Bis((Trifluoromethyl)Sulfonyl)Imide. Expanded use in battery technology and resource extraction responds to rising demand for efficient, low-waste processes. As regulatory bodies impose stricter controls on volatile or persistent chemicals, the appeal of non-flammable, low-toxicity liquids only grows. Forward-looking R&D targets new blends or additive packages to tailor flow properties and solubility, answering the call from high-throughput chemical manufacturing and grid-scale energy storage. Environmental scientists track breakdown pathways, steering regulators and designers toward use-and-recovery strategies that keep releases in check. By blending practical chemical know-how with active evaluation of lifecycle impacts, prospects for this compound look strong as industries push the bounds of clean tech and resource circularity.
Pulling Back the Curtain on a Mouthful of a Chemical
Tributylhexylphosphonium bis((trifluoromethyl)sulfonyl)imide—what a name. Those who work with chemicals or specialize in energy research will find this compound familiar, although it rarely turns up in everyday conversation. Its niche may not catch much popular attention, but the industries that use it know why it matters. I’ve seen my share of technical names in my years covering tech and materials science, but this one stands out for the ground it covers.
Why Do Scientists Reach for This Chemical?
The real kicker here is that this chemical falls in the category of ionic liquids. These liquids don’t behave the way most solvents do. They don’t evaporate quickly, and they can handle high temperatures and still remain stable. Because of that, researchers like to use these liquids for batteries, fuel cells, and certain forms of advanced manufacturing. In labs, chemists often search for materials that won't light up or degrade when pushed to extremes. This compound delivers by allowing processes to run hotter and longer. That feeds right into the push for safer, more durable parts in electronics and industrial machinery.
Helping Rechargeable Batteries Go the Distance
Some of the highest impact uses come down to lithium-ion batteries. Traditional batteries have always had a weak spot: their liquid electrolytes can catch fire or break down after too many cycles. The phosphonium-based ionic liquid changes things here. It replaces the usual electrolyte, making batteries less likely to overheat or burst into flames. Researchers at universities and corporate labs have tested this in prototype batteries and seen big improvements in both safety and lifespan. I’m always keen on advancements that translate to longer phone charge or electric cars with fewer recalls, and this material pushes the needle closer.
Greener Solutions for Chemical Processing
Industrial plants use solvents in almost every step, from extracting valuable metals to creating pharmaceutical ingredients. Most solvents come with drawbacks—probably flammable, sometimes toxic, often hard to recycle. Tributylhexylphosphonium bis((trifluoromethyl)sulfonyl)imide offers a work-around. It dissolves tough materials without the same risks. As workers on the line look for ways to limit fires or nasty fumes in the air, this chemical stands out. The right solvent saves money on safety systems and cleanup, making operations smoother and more sustainable. There’s also less energy wasted, since lower volatility cuts cooling costs.
Staying on the Right Side of Regulation
Regulating agencies across the globe now pay closer attention to chemicals that fly under the radar. Phosphonium-based compounds have so far kept a clean record. No ugly stories about widespread contamination or chronic health impacts. Still, researchers and plant managers keep one eye on new tests and recommendations so any emerging risks don’t cause trouble. As more sectors adopt these materials, routine health and safety reviews should remain a priority. I’ve covered stories where companies paid for lax oversight, and nobody wants to see cutting-edge tools turn into tomorrow’s environmental headache.
Where Could Things Go From Here?
Engineers mix new chemicals into battery prototypes and test green solvents every year. Tributylhexylphosphonium bis((trifluoromethyl)sulfonyl)imide brings a rare set of properties that unlock options from energy storage to electronics to advanced extraction methods. The drive to replace petrochemical solvents and improve device safety keeps demand for better ionic liquids alive. As more researchers share data, expect the boundaries of what these compounds can do to expand. In my time reporting on tech, I’ve watched old limitations fall away as new materials change the rules. This one looks poised to follow that pattern.
Understanding the Real Risks
Nobody likes surprises in the workplace, especially when it comes to handling chemicals. Long hours in labs and warehouses have taught me that a moment's carelessness often brings regret. Spill something strong, forget your gloves, breathe in something strange—these aren't just minor slip-ups, they can turn a regular workday upside down.
Reading Labels Like Your Health Depends On It
Those labels with black diamond warnings aren’t just paperwork left over from the last inspection. They point to what can go wrong, and what you might suffer if you ignore their advice. Chemicals can burn, blind, poison, or trigger fires. Even everyday products like cleaning agents sometimes hide aggressive acids or solvents. Reading the safety data sheet saves trouble down the line. These sheets spell out what harms you—maybe an allergic reaction, maybe something that hits after years of exposure. The information tells you how to keep yourself safe, not just how to store the box.
Personal Experience: Don’t Cut Corners
In warehouses filled with barrels, most injuries I’ve seen come from skipping steps. I remember one guy ignoring the eye-wash station instructions because gloves felt too hot that summer day. He got splashed and spent the afternoon with his head stuck under running water. That incident taught everyone around him: shortcuts don’t just put you at risk, they pause the whole operation. Besides a scolding from the boss, he learned a valuable lesson about respecting the gear.
More Than Just Gloves and Goggles
Personal protective equipment matters, but it’s not just about wearing the thickest gloves or the fanciest masks. It’s knowing which kind to pick up for which job. Not every mask blocks chemical fumes. Not every glove stops acid. It pays off to match the protection to the danger. Changing gloves after spills, washing hands before eating, checking shoes for leaks—sounds simple, but this habit-building stuff makes a massive difference over years.
Proper Storage Keeps Everyone Safe
Back in the stockroom, a leaking cap nearly ruined packs stacked underneath. Storing chemicals off the floor and away from sunlight avoids unnecessary reactions and leaks. Separating acids from bases, fuels from oxidizers—this keeps the place from turning into a chemistry set gone wrong. Organizing bottles on sturdy shelves, away from heat and out of reach of untrained personnel, prevents accidents that no one wants to clean up.
Smart Disposal Saves People and the Planet
Pouring leftover chemicals down the drain or tossing containers into regular trash bins—these mistakes pollute water and make landfill workers sick. Proper waste bins and clear instructions on chemical disposal stop these issues. Local disposal rules can feel strict, but the cost of an environmental fine, or someone’s health, far outweighs the effort.
Training and Accountability Matter
Supervisors who run regular briefings and encourage questions build a safer culture. In my experience, people feel comfortable admitting confusion and reporting small leaks if the boss listens instead of barking orders. A simple sign-out sheet helps track who used what, and clear procedures let everyone know how to handle spills. No need to guess when help is needed, since everyone on site should know emergency contacts and first aid spots.
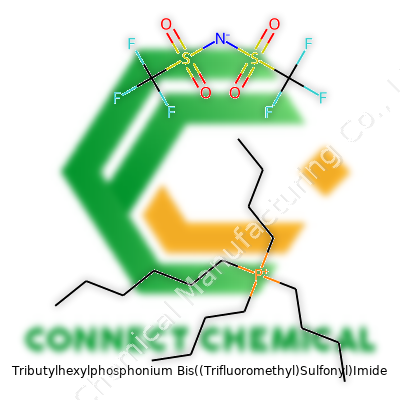
Chemical Structure and Formula Explained
Tributylhexylphosphonium Bis((trifluoromethyl)sulfonyl)imide has built a reputation in the world of ionic liquids, primarily due to its unique chemistry. Breaking it down, the core features a phosphonium cation where a phosphorus atom bonds with three butyl chains and a single hexyl chain. The formula for the cation reads as [P(C4H9)3C6H13]+. Each alkyl side chain pushes the molecule toward increased hydrophobic character, which matters in many applications where water content disrupts results.
Balancing this cation, the counterpart anion is Bis((trifluoromethyl)sulfonyl)imide, featuring two trifluoromethyl groups (CF3) and two sulfonyl groups (SO2), connected via a central imide nitrogen. The full shorthand for the anion looks like [N(SO2CF3)2]–. This anion plays a vital role by supporting the salt’s low viscosity, high thermal stability, and good solubility for a variety of organic solutes.
Bring these two ions together, and the formula reads: [P(C4H9)3C6H13][N(SO2CF3)2]. In the lab, people often refer to this as an ionic liquid, not just a fancy solvent. Thanks to strong ionic interactions, it stays liquid across a wide temperature range—a detail that enables a host of reliable processes, especially when water or traditional solvents bring too many hurdles.
Why Structure Matters in Everyday Research and Industry
During my graduate work, I often dealt with solvents that evaporated or decomposed right when my reactions got interesting. The moment I swapped in an ionic liquid like this one, reaction windows widened, and I could push temperatures without worrying over fumes or messy degradation. That’s exactly where the structure of Tributylhexylphosphonium Bis((trifluoromethyl)sulfonyl)imide matters most.
The longer alkyl chains hanging off phosphorus boost liquid range and reduce volatility. Unlike classic phosphonium salts, this combination cuts down on odor and presents fewer handling hazards. The bulky, flexible structure stabilizes the ions against breakdown, which means researchers can plan longer, more demanding experiments.
In commercial spaces, battery and electroplating industries count on ionic liquids to dissolve tricky salts or mediate exchange between electrodes. The robust combination of phosphorus and sulfonyl groups grants this compound resistance to oxidation and other forms of decay that cripple organic solvents under severe electric or thermal loads. No scientist enjoys swapping out broken equipment or pausing for leaks and contamination—using the right ionic liquid keeps those headaches in check.
Tackling Challenges in Scale and Environmental Impact
Yet, every industrial chemist knows that convenience comes with a responsibility. Ionic liquids, including this phosphonium salt, stir up concern around cost, synthesis complexity, and end-of-life fate. Once, after a series of nightlong reactions, I realized the headaches of disposal since some ionic liquids can persist in water. Being smart about their full life cycle—starting with greener synthesis, ending with better recycling or safe decomposition—calls for new partnerships between labs, regulators, and supply chains.
Shifting toward renewable feedstocks for both phosphonium and bis(trifluoromethylsulfonyl)imide parts can help. Companies can redesign synthetic routes to cut down waste or create closed-loop systems that recover these valuable salts after use. Open dialogue about toxicity and environmental performance, combined with daily trial-and-error by scientists, shapes safer norms. Staying curious and persistent sets the tone for next-generation solvents that solve today’s problems without leaving a scar for tomorrow.
Why Storage Matters More Than Folks Think
Lots of people barely glance at the storage instructions on products, but the place a product calls home changes everything about its quality. I’ve worked in a pharmacy and have seen drugs lose their punch just because someone kept them in a steamy bathroom cabinet. It doesn’t stop at pills. Food, cleaners, and even batteries tell the same story. All it takes is a little know-how to dodge loss and save money.
Heat and Humidity: The Twin Trouble-Makers
Ask any food worker or pharmacist, and most will point to heat and water in the air as the top reasons for stuff going bad too soon. Higher temperatures boost chemical reactions, and those reactions wreck shelf life. Humidity finds its way into pretty much anything—powders form clumps, electronics get corrosion, and even paints start to break down.
Some folks think tossing everything in the fridge solves the problem, but cold can bring its own mess. Products meant for room temperature get damaged by a dip in temperature or repeated cycling between hot and cold. Condensation sneaks in when you move a cold container to a room that’s warmer. Moisture wrecks all sorts of products, from flour to supplements.
Sunlight Destroys Quietly
Direct sunlight kicks off chemical changes in all sorts of items. I’ve seen vitamins fade from strong yellow to colorless, losing strength at the same time. People might line up their fancy skincare in a window for the look of it, but that light leads to breakdown and wasted money. UV rays seem harmless until you’re left with a product that doesn’t work as promised.
Smart Storage Isn’t Complicated
Simple steps carry the most weight. Keep products in their original containers since they’re usually built for protection. Clear containers look nice, but they let in more light. Closets, drawers, and cabinets offer safe spaces for most goods. I’ve always encouraged family to store pantry foods in dark, cool spots—top shelves near stovetops make things go stale quicker than you’d guess.
Some things thrive in dry spaces. A silica packet sometimes comes tucked inside a bottle for good reason. Save those if the product is prone to absorbing water. If you deal with items that have a strong smell, like cleaning chemicals, keep them tightly sealed and away from food storage. Mixing smells and chemicals can create real hazards.
Checking Expiry and Common Sense Tips
Every product I’ve kept in the right spot lasted longer and saved me from extra trips to the store. If something is labeled “store below 25°C,” treat that as solid advice. If a label says “keep away from sunlight,” look for a spot where the sun doesn’t reach. When in doubt, avoid damp spaces. An attic or garage might seem fine, but temperature swings speed up decay.
Separation is smart, too. Don’t mix products that react with each other. Keep cleaning agents and medications apart. If you aren’t sure about a product’s needs, most manufacturers offer advice online or with their customer service hotlines. Reliable information prevents waste and frustration.
Taking Storage Seriously
It boils down to respect for what you buy. Good storage isn’t only about science—it’s about taking care of your health and your wallet. Use what you’ve got: labels, cool closets, sturdy containers, and a little bit of attention. The reward shows up every time you reach for something that actually works the way it should.
Looking Past the Name: Why Chemists Care About Compatibility
Long chemical names have a reputation for scaring off everyone except lab geeks. Tributylhexylphosphonium bis((trifluoromethyl)sulfonyl)imide—usually shortened to TBHP-TFSI—falls into that bucket. Still, for people working with materials science, advanced batteries, or green chemistry, the chemical mix in the flask can mean the difference between progress or a hazardous, expensive mess. Compatibility isn’t just about avoiding explosions. It comes down to safety, money, cleanliness, and real-world results.
What Makes TBHP-TFSI Special in the Lab
I’ve worked with ionic liquids for years, and TBHP-TFSI belongs to that club. Chemicals like this one are unique; their liquid state at room temperature lets them replace volatile organic solvents. TBHP-TFSI has these huge, oil-like molecules that don’t evaporate easily. This helps keep the workplace air cleaner and cuts back on toxic waste. You’d think something so stable would get along with just about anything, but nature’s rarely that simple.
Speed Bumps: What Happens Mixing with Common Solvents?
People expect ionic liquids to blend into organic solvents without a hitch. That’s not always true. In my lab, we poured TBHP-TFSI into polar solvents like acetonitrile, expecting it to mix smoothly. The two created stubborn layers. That came from the chemical structure—TBHP-TFSI’s ions carry a bulky, greasy tail. Those tails avoid contact with water and many alcohols. In contrast, the same salt handles non-polar solvents with more flexibility.
Companies tout TBHP-TFSI as a “universal solvent,” but try stirring it into something like DMSO or methanol. The mismatched polarity makes full mixing a headache. Chemists ran tests showing water tolerance ranges from almost none to about five percent, based on temperature and what else floats in the mix. Even a small difference here can clog up a process, slow down purification, or cause headaches cleaning equipment.
Why Mixing Matters to Industry and Research
Compatibility guides every step after buying a fresh bottle. Nobody wants a failed reaction because the ionic liquid pushes the catalyst out of solution. I’ve seen teams waste entire days trialing different solvent combos—hoping for clear solutions, only to get cloudy messes or unseparated layers. Battery engineers face similar problems as they hunt for salts that dissolve evenly and keep devices stable under charge and discharge cycles.
What To Do Differently
Some scientists check compatibility charts—that’s a step, but many tables skim over newer compounds. Real progress comes with a shift in thinking. Instead of grabbing “standard” solvents, folks should run down basic questions: What happens if I change the temperature by a few degrees? What’s the outcome if I throw in a cosolvent? How will impurities affect stability over time?
Labs trying to avoid repeated dead ends can share data. Everyone benefits when negative results get published. Open data helps future researchers skip the mistakes and focus on tweaking concentrations or temperatures rather than repeating basic work. Companies embracing this model can save resources, speed up commercialization, and keep employees safer.
Final Thoughts From the Bench
Every new chemical, including TBHP-TFSI, brings hope for cleaner processes and better products. But ignoring how it mixes with the world’s existing chemicals can bog down even the best ideas. The science works best when chemists build on shared experience—not just chemistry in isolation, but chemistry as a team sport.