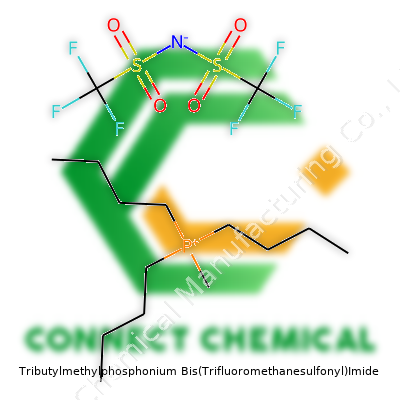
Tributylmethylphosphonium Bis(Trifluoromethanesulfonyl)Imide: A Deep Dive
Historical Development
Scientific curiosity has always driven chemists to search for better ionic liquids, hoping to land a combination that really opens up new ways of doing chemistry. Tributylmethylphosphonium Bis(Trifluoromethanesulfonyl)Imide, known in labs as [P4441][NTf2] or TBMP-TFSI, sprang from that ongoing work. The late 20th century saw a rush to create task-specific ionic liquids. Researchers had already worked with imidazolium and pyrrolidinium salts, but phosphonium-based ionic liquids promised better thermal stability for demanding industrial processes. By the 2000s, labs in Germany, the UK, and Japan picked up on this new substance’s promise. Technical papers soon outlined its preparation, usage, and advantages over predecessors—always addressing concerns like purity and water content, which can really affect experimental results.
Product Overview
Tributylmethylphosphonium Bis(Trifluoromethanesulfonyl)Imide stands out in the ionic liquid family, mainly because it shows remarkable stability and remains a liquid at room temperature. Chemists appreciate its wide electrochemical window and low volatility, both points that matter in air- and moisture-sensitive applications. Unlike many liquids, you won’t see it evaporating into the hood or releasing strong odors. This trait actually makes it a great choice for battery electrolytes, extraction solvents, and as a reaction medium where both temperature and chemical robustness matter. Many laboratories, mine included, switch to [P4441][NTf2] for experiments involving highly reactive intermediates or metals that otherwise behave unpredictably in conventional solvents.
Physical & Chemical Properties
This compound looks clear and colorless but packs plenty of punch in its properties. With a melting point well below room temperature, it flows freely across a range of conditions, even at moderate sub-zero temperatures. The density comes in at about 1.2-1.3 g/cm³ depending on the supplier, which is fairly typical for phosphonium ionic liquids. The viscosity stands out: it’s thicker than water but still handleable with syringes for precise dosing. Its high chemical and thermal stability—resisting breakdown even at 300°C—makes it a smart pick for high-temperature synthesis or heat transfer fluids. From firsthand experience, though, you always notice the slight slick, almost oily feel of an ionic liquid like this—so gloves should never be optional. Its hydrophobic nature means you don’t see it mixing with water quickly, which helps in separating products or cleaning up a reaction after everything settles down.
Technical Specifications & Labeling
Suppliers label TBMP-TFSI by chemical name, empirical formula, and molecular weight (usually 586.58 g/mol for the anhydrous form). The Certificate of Analysis details purity (commonly above 98%), water content (below 200 ppm), and traces of inorganic or organic impurities. Many labs request materials with known halide content, as even a few ppm can interfere with electrochemical measurements. Most containers come with batch numbers, recommended storage (sealed, away from light and moisture), and the date of production. Standard Safety Data Sheet (SDS) sheets highlight all the critical points—fire risk, spill response, handling recommendations—right down to the right type of gloves and goggles.
Preparation Method
Commercial supply firms prepare this ionic liquid by exchanging tributylmethylphosphonium bromide with lithium bis(trifluoromethanesulfonyl)imide in dry organic solvents, usually acetonitrile or dichloromethane. As with many ionic liquid syntheses, the real challenge lies in thoroughly washing out side products: lithium bromide, for instance, can stubbornly persist if you don’t wash with enough deionized water. Precise phase separation and repeated extraction allow collection of the organic phase containing the desired ionic liquid. Drying over molecular sieves removes residual water, while careful distillation gets rid of any unwanted solvent traces—no one wants leftover acetonitrile clouding up high-precision tests. The entire process, from a bench chemist's point of view, often involves iterative purification and long, patience-testing hours waiting for phase layers to settle.
Chemical Reactions & Modifications
[P4441][NTf2] shows stability against most acids and bases under normal conditions, holding up well in both organic and electrochemical transformations. The NTf2- anion sits largely inert, which keeps unwanted side reactions at bay, providing a reliable reaction medium. Its hydrophobicity lends itself to extractions and separations of nonpolar compounds—something I’ve seen work beautifully in isolating tricky organometallic complexes. In electrochemical applications, phosphonium’s robustness against decomposition means you don’t have to swap out your electrolyte as often, and performance remains steady over long testing runs. Some research groups look at swapping the methyl or butyl groups with functional groups that will impart new selectivity or coordination ability, so future variants could unlock even more possibilities for catalysis or sensing.
Synonyms & Product Names
On chemical catalogues, you’ll come across names like tributyl(methyl)phosphonium bis(trifluoromethylsulfonyl)imide, TBMP-TFSI, or [P4441][NTf2]. CAS numbers can help track down the exact variant—information that proves valuable for compliance records or grant paperwork. Some vendors extend the product line with branded labels or offer “ultra dry” or “high purity” variants for sensitive research projects, but the base chemical remains unchanged.
Safety & Operational Standards
Industrial safety teams regularly review protocols for these ionic liquids. Gloves—nitrile preferred—keep skin contact to a minimum, since the long-term effects remain less well studied compared to more common solvents. Eye protection, fume hoods, and labeling all matter. Any accidental spill means mopping up with plenty of absorbent and washing down tools with organic solvents before a final water rinse. Waste needs clear labeling and correct storage before disposal with licensed chemical waste contractors. I still remember hearing from a colleague how improper cleanup once clogged a shared glovebox filter; it took hours and ran up unnecessary costs. Fire risk stays low due to poor volatility, but one should never overlook good storage and daily documentation, as most universities require for new or advanced synthetic chemicals.
Application Area
Researchers often choose this liquid for electrochemistry, battery research, and catalysis when classic solvents don’t stand up to high-voltage gradients or aggressive reactants. The hydrophobic nature and stable ionic pairing (phosphonium cation and NTf2 anion) set it apart from imidazolium variants in moisture-sensitive syntheses. Some pharma labs turn to [P4441][NTf2] for Eastman or Suzuki-type couplings under “green” conditions. I’ve met battery developers who appreciate its window of chemical stability, making it a prime candidate for test cells aiming at better safety and higher charge/discharge rates.
Research & Development
Development teams in universities and industry alike keep tinkering with new derivatives. Much of the research investigates modifications to cationic or anionic partners, always searching for better conductivity, reduced viscosity, or higher miscibility with organic feedstocks. Teams evaluating fuel cells run comparative tests on phosphonium-based ionic liquids, often publishing data that pushes refinement of synthetic routes, cost reductions, and recycling pathways. Progress in computational modeling gives predictive power that wasn’t possible twenty years ago, and having used those tools myself, I can say it saves weeks of trial-and-error.
Toxicity Research
Toxicity still raises questions, and responsible labs don’t ignore preliminary results. Early work showed that phosphonium cations can persist in aquatic environments longer than ammonium analogs, raising flags for regulators. Some studies point to moderate toxicity toward Daphnia and fish, but less pronounced effects on plants and soil bacteria. Animal toxicity trials remain few, but researchers recommend using containment and strict segregation of workspaces as a precaution. This focus on environmental and personnel safety echoes across industry roundtables and conferences. As green chemistry gathers public attention, one can expect more funding and tighter controls around these substances.
Future Prospects
Growth looks set to continue, especially as energy storage and specialty fine chemical manufacture expand. As studies address current knowledge gaps—environmental persistence and real-world toxicity—new protocols will emerge, shaping best practices. I see opportunities for more bio-based or easily recoverable ionic liquids to emerge, nudged forward by both legislation and industry partnerships. Meanwhile, computational chemistry, automation, and advanced purification systems will make smart design and quick screening part of everyday development, much as high-throughput methods transformed drug discovery. TBMP-TFSI itself looks poised to feature in pilot scale-up work, helping close the gap between discovery and widespread industrial adoption.
Changing What We Know About Solvents
Researchers who spend time in the lab know that not every solvent can handle modern chemistry’s demands. The hunt for safe, stable, and practical fluids keeps pushing the envelope. Tributylmethylphosphonium bis(trifluoromethanesulfonyl)imide stands out. It doesn’t catch fire, doesn’t evaporate easily, and it allows reactions to run at higher temperatures without falling apart. That counts for a lot when you want to do something tricky—like synthesize pharmaceuticals or new electronic materials—without worrying about dangerous vapors or losing half your product with every stir.
Batteries Are Hungry for Better Liquids
Ionic liquids like this one have become a bit of a lifesaver for folks working on next-generation batteries. They serve as electrolytes that push ions back and forth between electrodes. The usual liquid electrolytes make engineers nervous because they can catch fire or break down after too many cycles. Adding tributylmethylphosphonium bis(trifluoromethanesulfonyl)imide helps with safety and can extend battery lifespans. Plus, it stretches the voltage window, letting designers build stronger, longer-lasting batteries for electric bikes, mobile devices, and power tools.
Far from Just a Lubricant: An Electrochemical Standout
Electrochemistry isn’t just for making car batteries or cleaning metals anymore. This chemical lets researchers rethink the possibilities with supercapacitors and fuel cells. Old-fashioned organic liquids leak or decompose under harsh conditions. Ionic liquids like tributylmethylphosphonium bis(trifluoromethanesulfonyl)imide can handle voltage abuse and sloppy conditions, letting new technologies charge faster and work in tougher spots—whether that’s underground sensors or satellites fighting wild temperature swings.
Some Help in Extraction and Recovery
People working in metal recovery and chemical purification deal with mixtures that aren’t easy to separate. Tributylmethylphosphonium bis(trifluoromethanesulfonyl)imide brings a handy set of solvent properties that can pull out rare earths, copper, or even precious metals from industrial waste. The process makes it simpler to reclaim valuable materials safely, cutting waste and saving money on raw resources. This helps close the loop in recycling, which feels more and more urgent these days as supply chains keep getting stretched.
Cleaner Catalysis—Without the Mystery By-Products
Chemical manufacturers need reactions to be reliable and clean if they want to avoid headaches during purification. Traditional solvents might leave by-products that are hard to deal with or that create regulatory problems. Switching to ionic liquids like this one reduces the risk of surprise reactions, and since it resists evaporation, plants waste less and pollute less. Companies stick with what works, and when cleaner chemistry is involved, regulators, workers, and neighbors all stand to benefit.
Ideas for the Future
Of course, every new solution brings a new challenge. Ionic liquids can carry a high price tag, and questions about what happens when they finally do break down remain. Researchers can keep working on bio-based routes and recycling schemes to make the process greener and the material cheaper. More collaboration across academia and industry could speed up breakthroughs and get these new technologies onto everyone’s workbench, not just the most well-funded labs.
Why Stability Matters Beyond the Lab
Shelf life goes far beyond how long something can sit on a warehouse shelf. I worked in quality control at a pharma plant, and every conversation about stability instantly turned practical. Stability means the product still works as intended months or even years after manufacturing. It means it’s safe, too. Once, I watched a shipment of tablets get trashed after stored too warm for just a week—no one trusted their potency after that. Products have to survive not just time, but the real conditions they’ll actually face.
How Ingredients Shape Shelf Life
Every ingredient brings its own personality to the table. Some stay rock solid, others throw fits at the smallest whiff of humidity. Aspirin, for example, breaks down into acetic acid if exposed to moisture, giving off that telltale vinegar smell. Common salt stays stable almost anywhere. Vitamins, especially C, break down just from the light they face during transport—no surprise fruit juice loses its punch after a few weeks open in the fridge.
Temperature, Light, and Oxygen: The Triple Threat
Heat usually speeds up chemical reactions, and not often in ways you want. Pharma and food factories keep warehouses cool for a reason. Storing most liquids above room temperature tends to shorten their safe window. Light pushes things along, too. In the lab, we watched colored solutions fade right before our eyes on sunny days, proof that some molecules just can’t handle photons.
Oxygen brings plenty of headaches. Lotions and oils go rancid because fats oxidize. Powdered food and drugs get packed in airtight containers, and sometimes little packets of oxygen-absorber get tossed in for extra caution.
Real Testing Beats Theoretical Promises
Regulations demand stability studies because shortcuts put people at risk. Accelerated aging tests use hot ovens, humidity chambers, and UV cabinets to predict how fast things break down in months instead of years. But nothing beats pulling old stock from storage and testing the batch—real-life can surprise you. Once, a cough syrup formula seemed solid in test conditions, but the coloring turned weird shades after a year in regular warehouse lighting. Turns out, exposure to warehouse lamps over time tripped a reaction nobody noticed in the lab. The manufacturer ended up changing the packaging to block that spectrum.
What Customers Can Do
Folks storing products at home face a simpler version of lab trials. I tell friends: keep caps tightly closed, avoid hot cars, and ignore use-by dates at your own risk. A bottle stored cool, dry, and upright lasts longer than one left on a sunlit windowsill. Check for weird smells, loss of color, or odd textures. These signals mean it’s time for a replacement—no fancy equipment required.
Better Packaging and Honest Guidelines
Manufacturers have already started using smarter packaging. UV-blocking bottles, vacuum-sealed pouches, and silica gel packets don’t just look good—they can double or even triple the practical shelf life. Still, it comes down to honest, clear information. Labels that actually say “store below 25°C and keep out of sunlight” keep their promises far better than decorations about “freshness guaranteed.”
Understanding the Stakes
I’ve opened dusty brown bottles in university stockrooms, found faded hazard labels, and caught that icy note of strange chemicals that makes the back of your neck tingle. Not all compounds demand the same respect in storage, but some promise big trouble if you lapse in focus. Tributylmethylphosphonium Bis(Trifluoromethanesulfonyl)Imide falls into that bucket where familiarity and caution need to walk side by side. This isn’t a bottle you want left open on any old shelf.
Keeping It Cool and Dry
Moisture doesn’t play nice with this salt. Even if the bottle seems sealed, humidity in the room can creep through slight cracks, bringing with it the chance for slow, unseen reactions. Chemists I know keep such compounds in well-sealed glass containers. They double-check the cap, wrap the rim with Teflon tape if the thread looks worn, and sometimes stash the whole thing in a desiccator. Dry conditions help prevent slow hydrolysis and unwanted functional group changes.
Heat is another enemy. Many ionic liquids carry the label “thermally stable,” but instructions usually urge storage under room temperature. That doesn’t mean an unventilated metal-cabinet baking in July heat. I store these salts on a shaded shelf, away from any hot water lines or sunlight patches. If your lab gets warm, a ventilated chemical storage fridge at around 5°C helps control long-term risks.
Respect for Chemicals That Don’t Smell
A big reason for storing this phosphonium salt properly: it doesn’t announce problems with a foul odor or changing color. Potential impurities creep in quietly, so it pays to label each bottle with both opening and recapping dates. Having spent afternoons chasing weird results in experiments, only to realize a months-old batch got damp, I can tell you skipping labels complicates every future experiment.
Real Safety Matters
Gloves and goggles serve as reliable friends every time you handle this chemical. Organic phosphonium salts don’t burn like petrol, but they do bring risks. Spills on skin may cause reactions that unfold slowly—nothing dramatic, sometimes redness hours later. I keep a spill kit within arm’s reach, with sodium carbonate or other neutralizing powders suited for acidic or sulfonyl spills. Safety data sheets flag this chemical as harmful if swallowed or inhaled, so even careful labeling and cold storage aren’t enough on their own—training counts.
Better Storage Practices for Future Labs
Labs with strong safety records usually keep a central log. Every person taking out or returning the bottle signs and adds a quick note about condition and quantity. This system means others know if the material ran low, or if the lid looked damaged. I’ve seen small stickers added to the shoulder of the bottle: one for “dry,” another for “refrigerated,” and another for “hazard class.” It helps even seasoned techs spot trouble without delay.
Final Thoughts on Responsible Chemical Management
Ionic liquids like Tributylmethylphosphonium Bis(Trifluoromethanesulfonyl)Imide make modern chemistry possible, but safe storage builds trust between the science and the researchers. Good habits protect the next set of hands reaching for the bottle, and keep the science honest. A dry, cool, clearly labeled, and well-documented container means your next reaction only surprises you with its results, not its risk.
The Difference Between Caution and Complacency
Anyone who’s ever worked in a lab, a warehouse, or even just read a label on a household cleaner knows: chemicals don’t forgive mistakes. It takes more than just reading a safety data sheet once—you have to pay attention every time. In my own experience, the difference between a minor spill and a serious emergency usually comes down to keeping your head in the game and following protocols every single time, even when you think you’ve got it memorized.
Personal Protective Equipment Isn’t Optional
Gloves and goggles might feel cumbersome when you first put them on, but one splash to the eye or burn on the skin leaves a memory that sticks. Common PPE includes lab coats, nitrile or latex gloves, and tightly fitted goggles. Respirators may be important if a chemical gives off hazardous fumes or dust. Even regular handwashing before and after handling cuts down on risks nobody wants to take home.
Ventilation Makes a World of Difference
Poor airflow in a workspace can quickly turn a manageable spill into a dangerous buildup of vapors. Good ventilation helps lower the risk of accidental poisoning. In labs I’ve spent time in, fume hoods always ran during handling, even for what seemed like minor procedures. It isn’t just about comfort. Chronic exposure—even to low levels—chips away at health before you notice.
Read the Label, Then Read It Again
One of the most basic steps gets skipped too often. Every chemical container should be clearly labeled with its name, concentration, and hazard icons. Double-checking the label before pouring or mixing saves more trouble than any emergency shower.
Storage: Out of Sight Isn’t Out of Mind
Chemicals like acids and bases don’t mix well together, literally. I learned early on that storing incompatible substances side by side means gambling with reactions that can cause fires, toxic fumes, or even explosions. Flammables belong in safety cabinets, and corrosives demand their own space. Never assume clear liquids are harmless—one forgotten bottle in the wrong place can lead to evacuation drills nobody wants to repeat.
Spill Preparedness Pays Off
Every space where chemicals get used needs clear protocols for spills. Spill kits have to be stocked and ready—neutralizing powders, absorbents, and proper disposal bags all go a long way toward turning a bad day into a manageable situation. Training isn’t a one-shot deal; ongoing refreshers keep everyone sharp, especially new team members who haven’t seen a real emergency yet.
Waste Disposal: One of the Unskippable Steps
No chemical should ever go down the drain unless the safety data sheet and local rules give the green light. Dedicated disposal containers cut down on accidents and legal headaches. Some places use color-coded buckets; others lock disposal rooms. What matters is making sure nobody guesses or takes shortcuts. In the long run, following protocols protects not just co-workers, but the local water supply and soil, too.
Training and Culture Build True Safety
Effective safety doesn’t just come from equipment or warning signs—it grows from a culture of respect and responsibility. Open discussions about close calls, regular drills, and keeping instructions easy to find all help. Everyone should feel empowered to speak up if something looks off. I’ve seen teams where looking out for each other saved more injuries than any rulebook ever could. The main lesson: there’s always value in double-checking the details.
Peculiarities of Chemical Purity in Research and Industry
Lab work often comes down to the quality of each chemical on the bench. In my time working with advanced materials, I've seen how the choice between different grades shapes projects from the very beginning. Tributylmethylphosphonium bis(trifluoromethanesulfonyl)imide, an ionic liquid with a name that’s tricky to say and even trickier to spell, also comes in a range of purities. The specific grade on offer often influences whether it lands in a university research lab or an industrial-scale process.
Distinction Between Common Grades
On the shelves of reputable suppliers, you’ll spot several versions. These include research-grade, analytical-grade, and sometimes technical-grade. Research-grade usually means a higher purity, in the neighborhood of 99% or better. Several synthetic methods produce variations in contaminants, and this affects the suitability for sensitive applications. Analytical-grade usually claims the lowest impurity levels and fits best in fields like electrochemistry, where traces of water or other substances play havoc on results.
Technical-grade is sometimes available too and often costs less. Still, it shows up mostly where absolute precision isn’t high on the checklist—think pilot plant runs, bulk testing, or non-critical solvent needs. In my own experience, skipping on purity in cases where reactivity or conductivity are critical, results in repeated failures and lost time.
Why Purity Trumps Price in Many Fields
The price tag sometimes seems almost punitive. Purity, though, always finds a way to reveal its value. In the world of battery research or organic synthesis, any suspicion about contaminants creeps into every troubleshooting session. Many teams—including one I worked with—once settled for a cheaper grade for a small pilot, which brought down overall costs. Yet, the downstream impact included equipment fouling and poor reproducibility, which meant more money and time went out the window. The lesson stuck with us.
Regulatory, Environmental and Safety Considerations
Regulations keep getting stricter, especially for chemicals with tricky elements like phosphorus and fluorine. Regulatory filings often require a certificate of analysis proving known purity levels and impurity profiles. Some purchasers face pushback if their paperwork misses details. Environmental workers monitoring emissions and disposal also check for trace components that could slip through with lower-grade chemicals. Inaccuracy here risks fines or worse. So, most companies seeking reliability stick with higher grades backed by detailed documentation.
Checking and Confirming Purity
It pays to look deeper than the datasheet. Reliable suppliers run extra analyses like NMR, Karl Fischer titration, or ion chromatography to check for water, halides, and related cations or anions. Some advanced applications go a step further, running in-lab confirmation by NMR or mass spectrometry, especially if even a subtle contaminant could throw off results or catalyze unwanted reactions. I remember a situation in a graduate lab where even tiny chloride contamination altered the entire course of an intended synthesis.
Paths Toward Better Solutions
Problems from mismatched purity requirements pop up fairly often. Cross-talk among suppliers, chemists, and quality assurance folks helps set expectations straight. Some companies work on custom purification solutions for hard-to-find grades with better traceability or batch-to-batch consistency. Others collaborate globally to refine production methods and documentation to satisfy new green-chemistry and regulatory standards. Chemists need a clear conversation with their vendor before the order goes out—detailing not just desired grade, but shipping details, storage, and stability over time. The right standard avoids headaches, and that lesson applies as much today as in the past.